







Estude fácil! Tem muito documento disponível na Docsity

Ganhe pontos ajudando outros esrudantes ou compre um plano Premium


Prepare-se para as provas
Estude fácil! Tem muito documento disponível na Docsity
Prepare-se para as provas com trabalhos de outros alunos como você, aqui na Docsity
Os melhores documentos à venda: Trabalhos de alunos formados
Prepare-se com as videoaulas e exercícios resolvidos criados a partir da grade da sua Universidade
Responda perguntas de provas passadas e avalie sua preparação.

Ganhe pontos para baixar
Ganhe pontos ajudando outros esrudantes ou compre um plano Premium
Comunidade
Peça ajuda à comunidade e tire suas dúvidas relacionadas ao estudo
Descubra as melhores universidades em seu país de acordo com os usuários da Docsity
Guias grátis
Baixe gratuitamente nossos guias de estudo, métodos para diminuir a ansiedade, dicas de TCC preparadas pelos professores da Docsity
Exercicios resolvidos cap.10
Tipologia: Exercícios
1 / 10

Esta página não é visível na pré-visualização
Não perca as partes importantes!







10-1. A Carnot refrigeration cycle absorbs heat at -12 C and rejects it at 40 C. (a) Calculate the coefficient of performance of this refrigeration cycle. (b) If the cycle is absorbing 15 kW at the -12 C temperature, how much power is required? (c) If a Carnot heat pump operates between the same temperatures as the above refrigeration cycle, what is the performance factor? (d) What is the rate of heat rejection at the 40 C temperature if the heat pump absorbs 15 kW at the - 12 C temperature?
Solution:
(a) Coefficient of performance = T 1 / (T 2 - T 1 ) T 1 = -12 C + 273 = 261 K T 2 = 40 C + 273 = 313 K Coefficient of performance = 261 / (261 + 313) Coefficient of performance = 5.02 - - - Ans. (b) Coefficient of performance = useful refrigeration / net work 5.02 = 15 kw / net work net work = 2.988 kW - - - Ans. (c) Performance factor = coefficient of performance + 1 Performance factor = 6.01 - - - Ans. (d) Performance factor=heat rejected from cycle/work required.
heatrejected 15kw
heatrejected Performance factor −
heatrejected 15kw
heatrejected
−
Heat rejected = 17.988 kw - - - Ans.
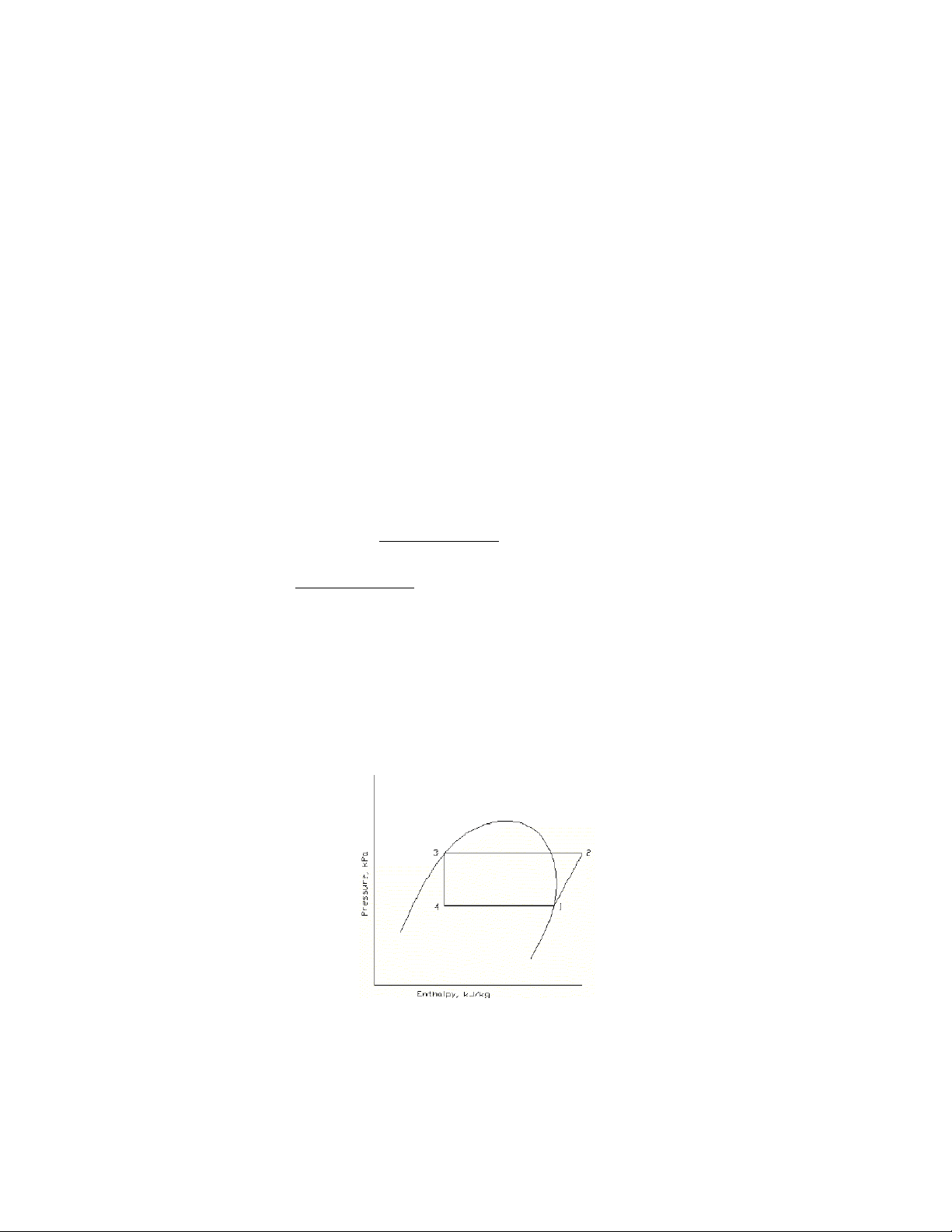
10-2. If in a standard vapor-compression cycle using refrigerant 22 the evaporating temperature is -5 C and the condensing temperature is 30 C, sketch the cycle on pressure-enthalpy coordinates and calculate (a) the work of compression, (b) the refrigerating effect, and (c) the heat rejected in the condenser, all in kilojoules per kilograms , and (d) the coefficient of performance.
Solution.
At pont 1, Table A-6, -5 C, h 1 = 403.496 kJ/kg
s 1 = 1.75928 kJ/kg.K
At point 2, 30 C condensing temperature, constant entropy, Table A-7.
h 2 = 429.438 kJ/kg
At point 3, Table A-6, 30 C h 3 = 236.664 kJ/kg
h 4 = h 3 = 236.664 kJ/kg
(a) Work of compression = h 2 - h 1
= 429.438 - 403. = 25.942 kJ/kg - - - Ans.
(b) Refrigerating effect = h 1 - h 4
= 403.496 - 236. = 166.832 kJ/kg - - - Ans.
(c) Heat rejected = h 2 - h 3
= 429.438 - 236. = 192.774 kJ/kg - - - Ans.
(d) Coefficient of performance
2 1
1 4 h h
h h Coefficient ofperformance −
Coefficientofperformance=
Coefficient of performance = 6.43 - - - Ans.
10-3. A refrigeration system using refrigerant 22 is to have a refrigerating capacity of 80 kw. The cycle is a standard vapor-compression cycle in which the evaporating temperature is -8 C and the condensing temperature is 42 C. (a) Determine the volume flow of refrigerant measured in cubic meter per second at the inlet to the compressor. (b) Calculate the power required by the compressor. (c) At the entrance to the evaporator what is the fraction of vapor in the mixture expressed both on a mass basis and a volume basis? Solution:
At 1, Table A-6, -8 C. h 1 = hg1 = 402.341 kJ/kg hf1 = 190.718 kJ/kg
For Dry Compression:
At 1, -20 C, Table A-3. h 1 = hg = 1437.23 kJ/kg hf = 108.599 kJ/kg s 1 = sg = 5.9025 kJ/kg.K sf = 0.65436 kJ/kg.K
At 2, 30 C Condensing Temperature, constant entropy, Fig. A-1. h 2 = 1704 kJ/kg
At 3, 30 C, Table A-3. h 3 = 341.769 kJ/kg s 3 = 1.48762 kJ/kg.K
At 4, s 4 = s 3 ,
5.9025 0.
s s
s s x g f
4 f = −
h 4 = hf + x(hg - hf) h 4 = 108.599 + (0.1588)(1437.23 - 108.599) = 319.586 kJ/kg
h h
h h Coefficientofperformance 2 1
1 4 −
Coefficient of performance = 4.
For Wet Compression:
At 2, 30 C condensing temperature, saturated, Table A-3. h 2 = 1486.14 kJ/kg s 2 = 5.2624 kJ/kg.K
At 1, s 1 = s 2.
s s
s s x g f
1 f = −
h 1 = hf + x (hg - hf) h 1 = 108.599 + (0.878)(1437.23 - 108.599) h 1 = 1275.14 kJ/kg
h 3 = 341.769 kJ/kg h 4 = 319.586 kJ/kg
h h
h h Coefficientofperformance 2 1
Coefficient of performance = 4.
Ans. 4.53 wet versus 4.19 dry.
10-5. In the vapor-compression cycle a throttling device is used almost universally to reduce the pressure of the liquid refrigerant. (a) Determine the percent saving in net work of the cycle per kilograms of refrigerant if an expansion engine would be used to expand saturated liquid refrigerant 22 isentropically from 35 C to the evaporator temperature of 0 C. Assume that compression is isentropic from saturated vapor at 0 C to a condenser pressure corresponding yo 35 C. (b) Calculate the increase in refrigerating effect in kilojoules per kilograms resulting from use of expansion engine.
Solution:
Vapor-Compression Cycle:
hb = 430.504 kJ/kg
At c, Table A-6. hc = 243.114 kJ/kg sc = 1.14594 kJ/kg
At d, constant entropy.
1.75279 1.
s s
s s x ga fa
d fa = −
hd = hfa + x(hga - hfa) hd = 200 + (0.193866)(405.361 - 200) hd = 239.813 kJ/kg
Net Work = (hb - ha) - (hc - hd) Net Work = (430.5 - 405.361) - (243.114 - 239.813) Net Work = 21.838 kJ/kg Refrigerating Effect = ha - hd = 405.361 - 239.813 = 165.548 kJ/kg
(a) Percent Saving
= 13.1 % - - - Ans. (b) Increase in refrigerating effect. = 165.548 kJ/kg - 162.247 kJ/kg = 3.301 kJ/kg - - - Ans.
10-6. Since a refrigeration system operates more efficiently when the condensing temperature is low, evaluate the possibility of cooling the condenser cooling water of the refrigeration system in question with another refrigeration system. Will the compressor performance of the two systems be better, the same, or worse than one individual system? Explain why.
Solution:
Coefficient of performance of two system:
w(h h) w (h h )
w(h h) w (h h) COP 1 2 1 a b a
1 1 4 a a d c (^) − + −
Coefficient of performance of each system
w(h h )
w(h h) COP 1 2 1
1 1 4 (^1) −
w (h h )
w (h h) COP a b a
a a d (^2) −
Substituting:
2
a a d 1
1 1 4
1 1 4 a a d c
COP
w (h h) COP
w(h h)
w(h h) w (h h ) COP −
if COP 1 = COP 2 then:
COPc = COP 1 = COP 2
Therefore it is the same COP as for individual system having equal COP and in between if COP is not the same..Ans.
10-7. A refrigerant 22 vapor compression system includes a liquid-to-suction heat exchanger in the system. The heat exchanger warms saturated vapor coming from the evaporator from -10 to 5 C with liquid which comes from the condenser at 30 C. The compressions are isentropic in both cases listed below. (a) Calculate the coefficient of performance of the system without the heat exchanger but with the condensing temperature at 30 C and an evaporating temperature of -10 C. (b) Calculate the coefficient of performance of the system with the heat exchanger? (c) If the compressor is capable of pumping 12.0 L/s measured at the compressor suction, what is the
(c) Refrigerating capacity without heat exchanger At 1, ν = 65.3399 L/kg
Refrigerating Capacity
65.3399L/kg
12.0 L/s −
65.3399L/kg
12.0L/s
= 30.3 kW - - - - Ans.
(d) Refrigerating capacity with heat exchanger At 1, ν = 70.2751 L/kg
Refrigerating Capacity
70.2751L/kg
12.0 L/s −
70.2751L/kg
12.0L/s
= 29.9 kW - - - - Ans.