














Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
The concept of chemical reactions, focusing on synthesis and decomposition reactions. Synthesis reactions involve the combination of two or more reactants to form a larger product, while decomposition reactions involve the breakdown of a larger reactant into smaller substances. examples and balanced chemical equations for each type of reaction.
What you will learn
Typology: Slides
1 / 22

This page cannot be seen from the preview
Don't miss anything!















Click on this icon for audio
There are millions of chemical reactions that are known to occur. Among these millions of reactions, certain types display similar characteristics. As a result, chemists are able to group reactions into 4 basic types to help organize these known reactions and to help chemists predict the products of unknown reactions. Click to learn about synthesis reactions Synthesis Decomposition Single Displacement Double Displacement Click on this icon for audio
In a synthesis reaction, two or more simpler reactants combine to form one larger product. The general formula for a synthesis reaction can be written as follows: Where A and B are the reactants, and they combine to form AB, the product. For a synthesis reaction to occur, the two reactant molecules must collide with each other, break any existing bonds between their atoms and form new bonds. Synthesis Reaction Example Synthesis Reactions^ Click on this icon for audio
A good example of a synthesis reaction occurs when diatomic molecules of hydrogen gas (H 2 (g) ) burn in the air, combining with diatomic molecules of oxygen gas (O 2 (g) ) to form water. Try to write out the balanced chemical equation for this reaction and you will see the general formula for a synthesis reaction represented. Balanced Synthesis Reaction Equation Synthesis Reactions^ Click on this icon for audio
Test your understanding: Which of the following reactions is a synthesis reaction? (Click the box with the correct answer.) 2HCl H 2
2
8
2
2
2
2
2
2
3 Synthesis Reactions
2HCl H 2
2
8
2
2
2
2
2
2
3
Synthesis Reactions
In a decomposition reaction, one large reactant compound breaks down (or decomposes) into two or more smaller substances. A decomposition reaction is the opposite of a synthesis reaction. Using this information, try to write a general formula for a decomposition reaction before clicking the button below. Click for the General Formula for a Decomposition Reaction Click on this icon for audio
In a decomposition reaction, one large reactant compound breaks down (or decomposes) into two or more smaller substances. The general formula for a decomposition reaction can be written as follows: Where the compound AB is the reactant which decomposes into the products A and B. More on Decomposition Reactions Decomposition Reactions Click on this icon for audio
Reactants in a decomposition reaction can decompose into simple elements, more complex compounds or combinations of the two. For example, potassium chloride (KCl) decomposes into solid potassium and chloride gas when electricity is used to provide energy. 2KCl (s) 2K (s)
More Decomposition Reactions Decomposition Reactions Click on this icon for audio
Decomposition reactions are used in many types of explosives such as TNT. Typically, for a decomposition reaction to occur, energy is needed to get the reaction started, this is why, for example, for a stick of TNT to explode a fuse must be lit. This fuse provides the energy to allow the decomposition reaction to begin, resulting in a flaming explosion. Pharaoh’s Serpent – Decomposition Reaction Decomposition Reactions Click on this icon for audio
A good example of a decomposition reaction is commonly referred to as the pharaoh’s serpent reaction in which a white powder made up of mercury thiocyanate is ignited resulting in a visually stunning decomposition reaction. Click for the Reaction Equation Decomposition Reactions Click on this icon for audio
In the pharaoh’s serpent reaction the large, complex molecule mercury thiocyanate, (Hg(SCN) 2 ) decomposes into carbon nitride (C 3
4 ), mercury sulfide (HgS) and carbon disulfide (CS 2 ) when energy is provided in the form of a flame or spark. Try to write the balanced equation for this reaction on your own, then click the button below to view it. Pharaoh’s Serpent Reaction Equation Decomposition Reactions Click on this icon for audio
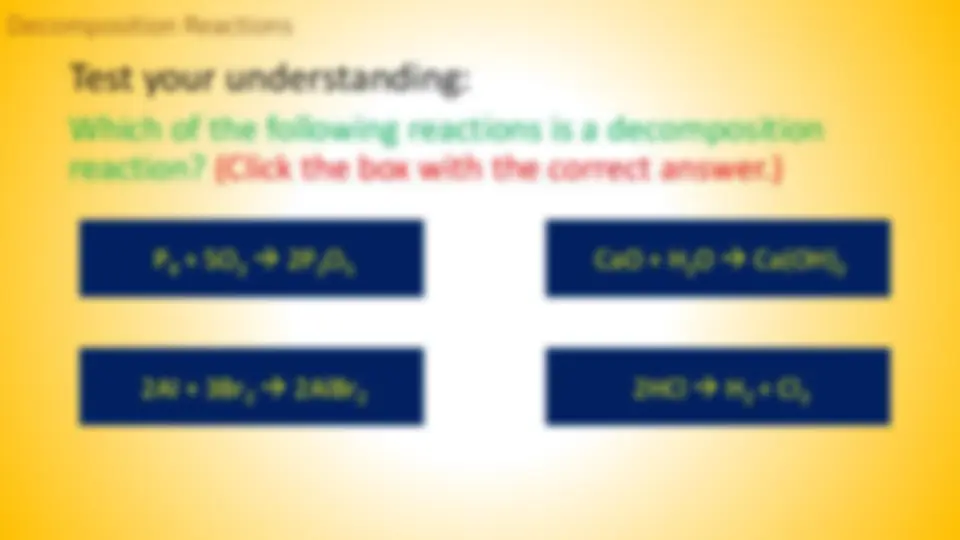
Test your understanding: Which of the following reactions is a decomposition reaction? (Click the box with the correct answer.) 2HCl H 2
4
2
2
5 Decomposition Reactions
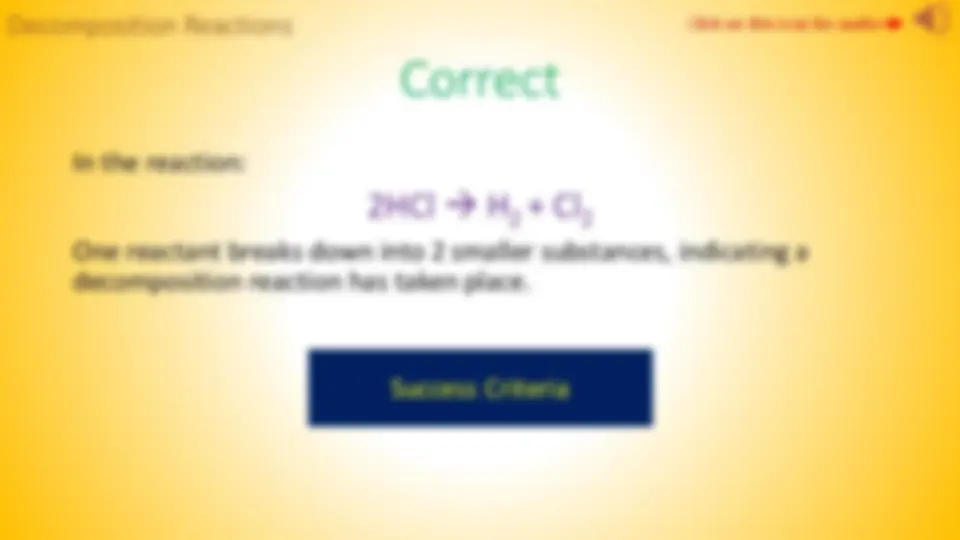
2HCl H 2
4
2
2
5
Decomposition Reactions