Download The Electron-Transport Chain - Lecture Slides | BIO 3390 and more Study notes Biochemistry in PDF only on Docsity!
• Biochemistry: A Short Course
• First Edition
• Biochemistry: A Short Course
• First Edition
Tymoczko • Berg • Stryer
© 2010 W. H. Freeman and Company
CHAPTER 19
The Electron-Transport Chain
electronsfrom NADH to O 2 , an exergonic process.
- (^) A molecular assembly in the inner mitochondrial membrane carries out the synthesis of ATP.
- (^) This enzyme complex was originally called the mitochon-drial ATPase or F 1 F 0 ATPase because it was discovered through its catalysis of the reverse reaction, the hydrolysis of ATP. ATP synthase, its preferred name, emphasizes its actual role in the mitochondrion. It is also called Complex V.
the transfer of electrons through the respiratory chain leads to the pumping of protons from the matrix to the cytosolic side of the inner mitochondrial membrane. The H+^ concentration becomes lower in the matrix, and an electrical field with the matrix side negative is generated called the chemiosmotic hypothesis, was that this proton-motive force drives the synthesis of ATP by ATP synthase.
- (^) ATP Synthase Is Composed of a Proton-Conducting Unit and
a Catalytic Unit
- (^) electron microscopic, and crystallographic studies of
ATPsynthase have revealed many details of its structure.
- (^) It is a large, complex membrane-embedded enzyme that
looks like a ball on a stick.
- The 85-Å-diameter ball, called the F 1 subunit, protrudes into
the mitochondrial matrix and contains the catalytic activity
of the synthase. In fact, isolated F 1 subunits display ATPase
activity. The F 1 subunit consists of five types of polypeptide
chains (αα 3 , β 3 , γ, δ, and ϵ) with the indicated stoichiometry.
- The α and β subunits, which make up the bulk of the F 1 , are
arranged alternately in a hexameric ring; they are
homologous to one another and are members of the P-loop
NTPase family.
- (^) Both bind nucleotides but only the β subunits participate
directly in catalysis. The central stalk consists of two
proteins: γ and ϵ. The γ subunit includes a long α-helical
coiled coil that extends into the center of the α 3 β 3 hexamer.
The γ subunit breaks the symmetry of the α 3 β 3 hexamer:
each of the β subunits is distinct by virtue of its interaction
with a different face of γ. Distinguishing the three β subunits
is crucial for the mechanism of ATPsynthesis.
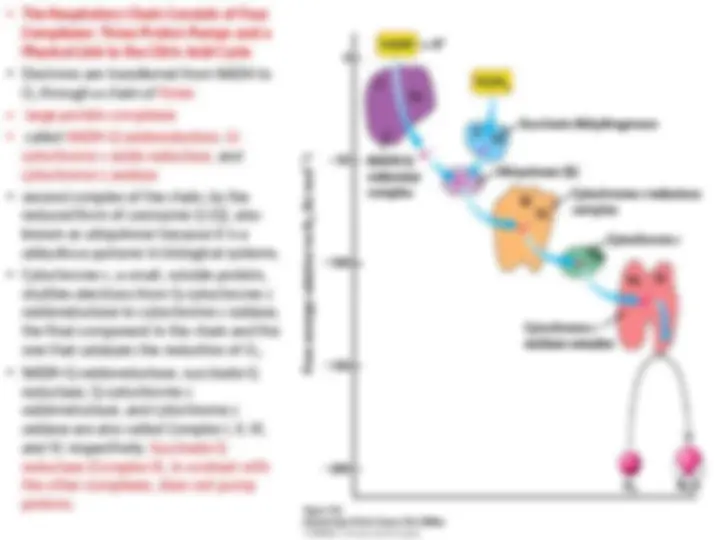
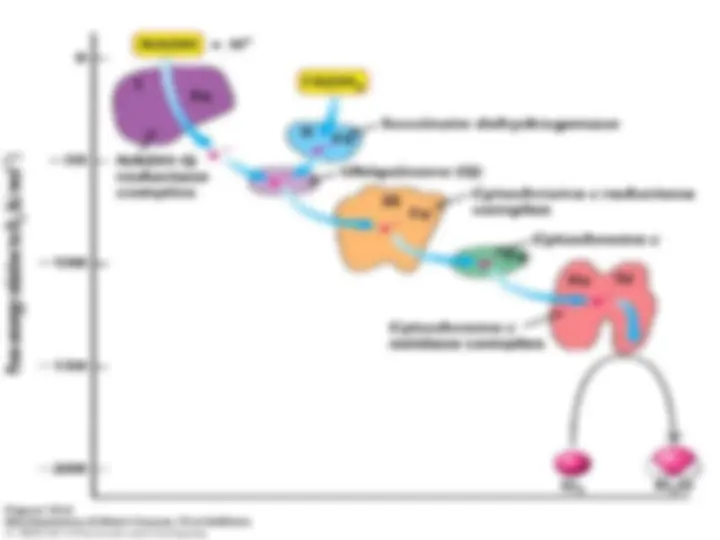
- (^) The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
- (^) Electrons are transferred from NADH to O 2 through a chain of three
- (^) large protein complexes
- (^) called NADH-Q oxidoreductase, Q- cytochrome c oxido-reductase, and cytochrome c oxidase
- (^) second complex of the chain, by the reduced form of coenzyme Q (Q), also known as ubiquinone because it is a ubi quitous quinone in biological systems.
- (^) Cytochrome c, a small, soluble protein, shuttles electrons from Q-cytochrome c oxidoreductase to cytochrome c oxidase, the final component in the chain and the one that catalyzes the reduction of O 2.
- (^) NADH-Q oxidoreductase, succinate-Q reductase, Q-cytochrome c oxidoreductase, and cytochrome c oxidase are also called Complex I, II, III, and IV, respectively. Succinate-Q reductase (αComplex II), in contrast with the other complexes, does not pump protons.