




































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
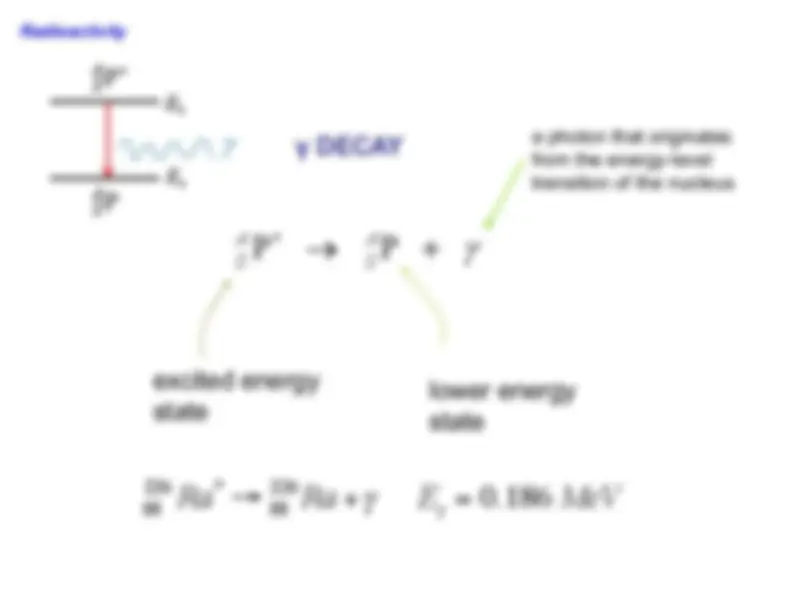
A comprehensive review of physics 1201, covering lectures 1 through 27. It includes key topics such as electric force, electric fields, electric potential, electric currents, dc circuits, magnetic forces and fields, electromagnetic induction, electromagnetic waves, reflection and refraction of light, interference, polarization, blackbody radiation, the photoelectric effect, the bohr atom, heisenberg uncertainty principle, and nuclear physics. The review also features examples and explanations of malus law, wiens displacement law, and the de broglie wavelength, making it a valuable resource for students preparing for their final exam. It also covers the mass deficit of the nucleus and nuclear binding energy. Useful for university students.
Typology: Exams
1 / 43

This page cannot be seen from the preview
Don't miss anything!




































à See Midterm 1 review (Lecture 12)
Polarization Example: Using Polarizers and Analyzers What value of q should be used so the average intensity of the po light reaching the photocell is one-tenth the average intensity of th unpolarized light?
Polarization
2 θ cos^ θ^ =^
θ = 63. 4 Applying Malus’ Law at the Analyzer: But and we want So I 0 I 0,polar I è è 𝐼 = 𝐼!,#$%&' cos 2 𝜃 I0,polar = ( ) I 0 𝐼 = 1 10 𝐼! 1 10 𝐼! = 1 2 𝐼! cos 2 𝜃
q Blackbody radiation
max
− 3 m ⋅ K
Example. a) Find the temperature of the Sun’s surface assuming that it emits blackbody radiation and the wavelength of the peak in its intensity distribution is at 500 nm (in the yellow region).b) Find the temperature of the star Betelgeuse (in the Orion constellation) which is classified as a Red Giant (near the end of its life cycle) with peak wavelength 890 nm. λ max
− 3 m ⋅ K T =
− 3 λ max
− 3 500 × 10 − 9
− 3 890 × 10 − 9 = 3260 K Much cooler than the Sun a) Sun: b) Betelgeuse: Blackbody radiation
Example. a) Find the energy of a photon with wavelength 210 nm. b) When light with this wavelength falls on a metal, the photoelectric circuit is brought to zero at a stopping voltage of 1.64 V. Find the work function of the metal. a ) E = hf = h c λ
− 34 ( )
8 ( ) 210 × 10 − 9 ( )
− 19 J = 5. 92 eV b ) KE max = e Δ V s
− 19 ( ) (^1.^64 )^ =^2.^62 ×^10 − 19 J = 1. 64 eV KE max = hf − W 0
0 = hf − KE max = 5. 92 − 1. 64 = 4. 28 eV The Photoelectric Effect
Example. What are the a) momentum and b) energy of a blue photon with wavelength 470 nm?
λ
− 34
− 9
− 27
− 27 ( )
8 ( )
− 19
The Photoelectric Effect
Example. What kinetic energy should neutrons (m = 1.67 x 10
− 34
− 24 kg m s KE = p 2 2 m
− 24
2 2 1. 67 × 10 − 27
− 20 J = 0. 0825 eV e.g. “thermal” neutrons from a nuclear reactor corresponding to a temperature of 640 o K KE = 3 2 kT k = Boltzman const. Set the neutron wavelength to be the interatomic spacing: l = 1.00 x 10
The Bohr Model of the Hydrogen Atom 1 , 2 , 3 , 2 = = n = h L mv r n n n n p ∴ r n = h 2 4 π 2 mke 2 "
$ % & ' n 2 Z n = 1 , 2 , 3 , … ( 5. 29 10 m) 1 , 2 , 3 , 2 11 = ´ =
Example: Find the wavelength of the second Balmer line in hydrogen, i.e. the n i = 4 to n f = 2 transition. 1 λ = ( 1. 097 × 10 7 m − 1 ) 1 n f 2 − 1 n i 2
$ % % & ' ( ( = ( 1. 097 × 10 7 m − 1 ) 1 2 2 − 1 4 2
$ % & ' ( =^2.^057 ×^10 6 m − 1 ⇒ λ = 486 nm blue The Bohr Model of the Hydrogen Atom
Example: Ionization of a hydrogen atom. What wavelength photon is needed to ionize a hydrogen atom in the ground state and give the ejected electron 11.0 eV of kinetic energy? KE = hf − E 1 hc λ
1 = 11. 0 + 13. 6 = 24. 6 eV = 3. 94 × 10 − 18 J λ = hc
− 34 ( ) 3.^00 ×^10 8 ( )