






Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Ozonolysis is a chemical reaction where ozone (O3) breaks the double bond of alkenes (olefins) to form carbonyl groups. an in-depth analysis of the reaction mechanism, including the formation of molozonides, zwitterions, and ozonides, as well as their reduction. The document also discusses the importance of ozonolysis as an analytical and synthetic tool, with a focus on the substitution patterns of acyclic and cyclic alkenes.
What you will learn
Typology: Lecture notes
1 / 10

This page cannot be seen from the preview
Don't miss anything!







Index: ● Overview ● Acyclic Alkene Substitution Pattern ● Resonance in Ozone ● The Molozonide ● Zwitterions ● The Ozonides ● Reduction of the Ozonides
● Ozonolysis is the process by which ozone (O 3 ) reacts with alkenes (olefins) to break the double bond and form two carbonyl groups. If the double bond of the alkene is substituted with hydrogen or carbon atoms, the carbonyl groups that are formed are either aldehydes or ketones. Acyclic alkenes form two carbonyl compounds while cyclic alkenes produce a single compound containing two carbonyl groups. As an analytical tool, ozonolysis reveals the substitution pattern of a double bond. Acting as a pair of chemical scissors, the reactive gas cuts the double bond and replaces it with oxygen atoms, i. e., carbonyl groups. However, ozonolysis does not afford information on the stereochemistry of the alkene if such stereochemistry existed originally. Thus, the generic, stereoisomeric alkenes 1 and 2 give rise to the same pair of carbonyl compounds. The astute reader will recognize that neither of these reactions is a balanced equation. Only two oxygen atoms (one equivalent of O 2 ) is required to balance this reaction. The fate of the third oxygen atom will be considered later.
Ozonolysis is also an important reaction from the synthetic perspective with compounds that have several functional groups. Because alkenes are nucleophilic and carbonyl groups are electrophilic, aldehydes and ketones can be stored as alkenes during synthetic reactions that are electrophilic. At the appropriate time, the alkene can be converted to the aldehyde or ketone when it is its turn to undergo electrophilic reactions. Acyclic Alkene Substitution Pattern: The substitution pattern of a double bond in an acyclic alkene can be ascertained by the number and type of carbonyl compounds that are formed (see Table below). If the two carbons of the double bond contain the same groups, then only one carbonyl compound is formed. Thus, ( E )- and ( Z )-2-butene give only acetaldehyde. If the two carbons of the double bond are substituted differently, then two carbonyl compounds are obtained. Double Bond Substitution Formaldehyde (CH 2 O) Aldehydes (RCHO) Ketones (R 2 CO) Unsubstituted (ethylene) 2 - - Monosubstituted 1 1 - 1,1-Disubstituted 1 - 1 1,2-Disubstituted - 2 - Trisubstituted - 1 1 Tetrasubstituted - - 2 Resonance in Ozone ● Ozone ( 3 ) is an oxide of oxygen. It is a resonance hybrid that functions in chemical reactions as though the terminal oxygens bear the positive and negative charge (box). (See the electrostatic potential map of ozone
There are two interesting points about the zwitterion. First, it may be viewed as an oxidized form of an aldehyde or a ketone. [Do you remember the extra oxygen in the beginning of the discussion? More later.]. Secondly, a zwitterion is also a resonance structure [see below]. In fact, it is isoelectronic with ozone itself. One of the terminal oxygens of ozone has been replaced with R-C-R to form the zwitterion. ● The Ozonides: You may now ask, "If the zwitterions is isoelectronic with ozone, shouldn't it react similarly?" It does! When the ozonolysis is conducted in a non-nucleophilic solvent such as CH 2 Cl 2 , the zwitterions 6 and 7 undergo cycloaddition reactions with the carbonyl compounds 5 and 8 to form ozonides 9 , 10 and 11 , each of which can exist as a pair of diastereomers (cis/trans). Thus, ozonide 9 can be formed from carbonyl 5 and zwitterion 6 and from zwitterion 7 and carbonyl 8. In addition, ozonide 10 arises from carbonyl 5 and zwitterion 7 while ozonide 11 forms from carbonyl 8 and zwitterion 6. In fact, each of the ozonides can form two stereoisomers as long as th R-groups are different. Six stereoisomers does not present a problem because the ozonides will be decomposed in a subsequent reaction. In the mechanism below, the red and green bonds and unshared electron pairs are the ones involved in ozonide formation.
Reduction of the Ozonides: The conversion of an alkene to an ozonide is a 6-electron oxidation yet the oxidation of an alkene to two carbonyl compounds is a 4-electron oxidation. A 2-electron reduction of the ozonides is required. In words, one of the oxygens of the ozonide must be reduced. The O-O bond of an ozonide has one of the oxygens more electron deficient than the other. The two electron reduction of the O-O bond of the ozonide is often accomplished with zinc or dimethyl sulfide. The mechanism below illustrates how the ozonide is reduced by two electrons while the dimethyl sulfde is oxidized by two electrons to dimethyl sulfoxide 13. Cyclic Alkene Substitution Pattern: The carbonyl compounds obtained from cyclic alkenes are the same as that obtained from acyclic alkenes except that both carbonyl groups are in the same molecule. That is, they are tethered together by the carbon chain that made the ring having an endocyclic (within the ring) double bond. Several examples follow:
way, if 19 and 23 are stereoisomers, then there must be a difference in the double bond, right? Finally, let's consider the ozonolysis of limonene, 13. Limonene contains an exocyclic and an endocyclic double bond. The exocyclic double bond accounts for the two products of ozonolysis, 25 and 26. The presence of formaldehyde 26 means that one of the double bonds in limonene is monosubstituted, i.e., formaldehyde correlates with the aldehyde group of 25 , or one of the double bonds is 1,1-disubstituted, i. e., correlation with one of the keto groups in 25. There are three permutations by which 24 can be reassembled to form an endocyclic double bond. The aldehyde group and the righthand keto group in 25 lead to limonene 24 , a 6-membered ring. The other two permutations lead to cyclopentene 27 and cyclobutene 28. It is impossible to decide without more information.
Discovering the Mechanism of Osmylation Osmylation of alkenes has become a critically important method of functionalizing an alkene due to two discoveries: 1) the reaction rate is accelerated by addition of amines that bind to Os; 2) use of chiral amines (particularly those derived from cinchona alkaloids such as quinine and quinolidine) allow not just high stereocontrol (syn addition), but also complete enantiocontrol — any absolute stereochemistry at the diol can be prepared based on the configuration of the double bond and the alkaloid ligand chosen. There is a mechanistic question — key to understanding what the ligand is doing — over whether this is a concerted reaction (both C-O bonds form at once), or whether the alkene binds to the metal and makes the C-O bonds in a 2-step sequence:
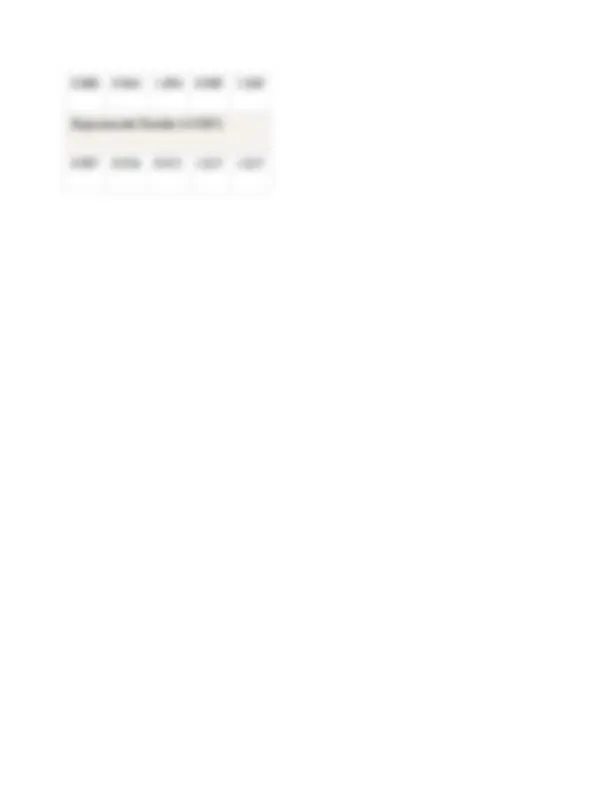
0.880 0.964 1.094 0.989 1. Experimental Results (± 0.005) 0.907 0.918 0.925 1.027 1.