


























Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
An insight into the properties of phospholipids that enable membrane formation and the distinct protein compositions of various membranes. It also discusses the ways membrane proteins interact with the lipid bilayer, their classification, and the differences between membrane and soluble proteins. The document also covers the role of integral and peripheral membrane proteins in energy transduction and membrane fluidity regulation.
Typology: Study notes
1 / 34

This page cannot be seen from the preview
Don't miss anything!



























© 2010 W. H. Freeman and Company
way is to form a micelle , a globular
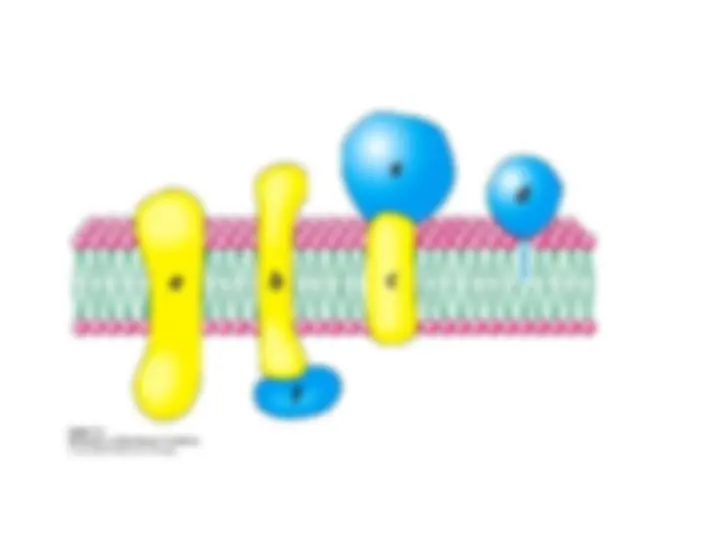
Membrane proteins can be classified as being either
Membrane proteins can be classified as being either
peripheral
peripheral or
or integral
integral on the basis of this difference in
on the basis of this difference in
dissociability.
dissociability.
Integral membrane proteins
Integral membrane proteins interact
interact extensively with the
extensively with the
hydrocarbon chains
hydrocarbon chains of membrane lipids, and so only agents
of membrane lipids, and so only agents
that compete for these
that compete for these nonpolar interactions can release
nonpolar interactions can release
them
them .
peripheral membrane proteins
peripheral membrane proteins are
are bound
bound to membranes
to membranes
primarily by
primarily by electrostatic and hydrogen-bond
electrostatic and hydrogen-bond interactions
interactions
with the head groups of lipids. These polar interactions
with the head groups of lipids. These polar interactions
can be disrupted by adding salts or by changing the pH.
can be disrupted by adding salts or by changing the pH.
bound to the surfaces of
bound to the surfaces of integral proteins
integral proteins , on either the
, on either the
cytosolic or the extracellular side of the membrane.
cytosolic or the extracellular side of the membrane.
Others are
Others are anchored to the lipid bilayer
anchored to the lipid bilayer by a covalently
by a covalently
attached hydrophobic chain, such as a fatty acid.
attached hydrophobic chain, such as a fatty acid.
Amino Acid Sequence of
Amino Acid Sequence of
Bacteriorhodopsin. The
Bacteriorhodopsin. The seven
seven
helical regions are highlighted
helical regions are highlighted
in yellow
in yellow and the
and the charged
charged
residues in red.
residues in red.
A Channel Protein Can Be Formed from
A Channel Protein Can Be Formed from Beta Strands
Beta Strands
Porin,
Porin, a protein from the outer membranes of bacteria
a protein from the outer membranes of bacteria
such as
such as E. coli
E. coli and
and Rhodo-bacter capsulatus
Rhodo-bacter capsulatus , Structures
, Structures
of this type are built from β strands and contain
of this type are built from β strands and contain
essentially no α helices
essentially no α helices
Embedding Part of a Protein in a Membrane Can Link It to
Embedding Part of a Protein in a Membrane Can Link It to
the Membrane Surface
the Membrane Surface
prostaglandin H
prostaglandin H
2
2
synthase-
synthase- -different
-different
role for α helices in protein-membrane associations. This
role for α helices in protein-membrane associations. This
enzyme catalyzes the conversion of
enzyme catalyzes the conversion of arachidonic acid
arachidonic acid
into
into prostaglandin H
prostaglandin H
2
2
.
. Prostaglandin H
Prostaglandin H
2
2
promotes
promotes
inflammation
inflammation and
and modulates gastric acid secretion.
modulates gastric acid secretion.
not largely embedded in the membrane but, instead,
not largely embedded in the membrane but, instead, lies
lies
along the outer surface of the membrane firmly bound by a
along the outer surface of the membrane firmly bound by a
set of α helices
set of α helices with hydrophobic surfaces that extend
with hydrophobic surfaces that extend
from the bottom of the protein into the membrane This
from the bottom of the protein into the membrane This
linkage is sufficiently strong that only the action of
linkage is sufficiently strong that only the action of
detergents
detergents can release the protein from the membrane
can release the protein from the membrane .
Thus, this enzyme is classified as an integral membrane
Thus, this enzyme is classified as an integral membrane
protein
protein , although it is not a membrane-spanning protein.
, although it is not a membrane-spanning protein.
Membrane Fluidity Is Controlled by Fatty Acid
Membrane Fluidity Is Controlled by Fatty Acid
Composition and Cholesterol Content
Composition and Cholesterol Content
transport or signal transduction
transport or signal transduction , depend on the
, depend on the
fluidity
fluidity of the membrane lipids
of the membrane lipids
The transition from the rigid to the fluid state occurs
The transition from the rigid to the fluid state occurs
rather abruptly as the temperature is raised above
rather abruptly as the temperature is raised above T
mm
the melting temperature.
the melting temperature. This transition temperature
This transition temperature
depends on the length of the fatty acyl chains and on
depends on the length of the fatty acyl chains and on
their degree of unsaturation
their degree of unsaturation
. saturated fatty acyl . saturated fatty acyl
residues favors the rigid state.
residues favors the rigid state.
On the other hand,
On the other hand, a cis double bond produces a bend in
a cis double bond produces a bend in
the hydrocarbon chain. This bend interferes with a
the hydrocarbon chain. This bend interferes with a
highly ordered packing of fatty acyl chains
highly ordered packing of fatty acyl chains , and so
, and so T
m
m
is lowered
is lowered .
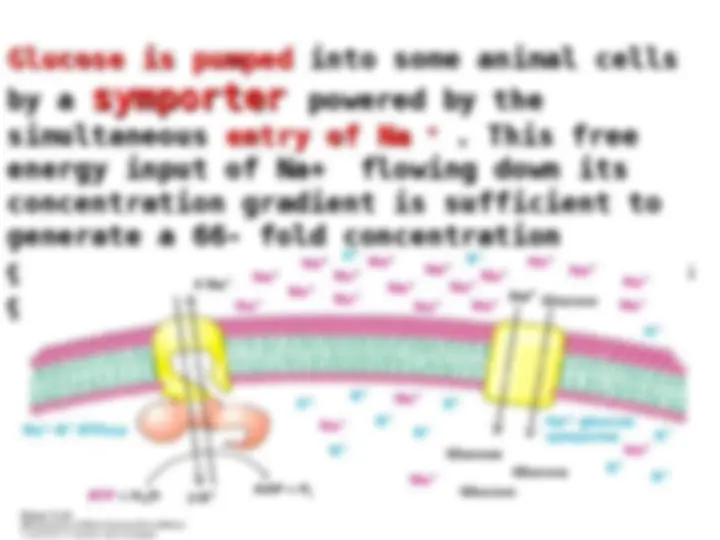
Na K pump – read page 164
Na K pump – read page 164
Na
Na
+
+
+
+
pump
pump or the
or the Na
Na
+
+
+
+
ATPase
ATPase
. The hydrolysis of . The hydrolysis of
ATP by the pump provides the energy needed for the
ATP by the pump provides the energy needed for the
active transport of Na
active transport of Na
out of the cell and K
out of the cell and K
into
into
the cell, generating the gradient. The pump is
the cell, generating the gradient. The pump is
called the Na
called the Na
ATPase
ATPase because the hydrolysis of
because the hydrolysis of
ATP occurs only when Na
ATP occurs only when Na
and K
and K
are bound to the
are bound to the
pump. Moreover, this
pump. Moreover, this ATPase
ATPase , like all such enzymes,
, like all such enzymes,
requires Mg
requires Mg
2+
2+
. The active transport of Na . The active transport of Na
and K
and K
is
is
of great physiological significance. Indeed, more
of great physiological significance. Indeed, more
than a third of the ATP consumed by a resting
than a third of the ATP consumed by a resting
animal is used to pump these ions.
animal is used to pump these ions. The Na
The Na
gradient in animal cells controls cell volume,
gradient in animal cells controls cell volume,
renders neurons and muscle cells electrically
renders neurons and muscle cells electrically
excitable, and drives the active transport of
excitable, and drives the active transport of
sugars and amino acids.
sugars and amino acids.
++
++
digitalis leads
digitalis leads
to a higher level of
to a higher level of
Na
Na
inside the cell.
inside the cell.
2+2+
Multidrug
Multidrug Resistance and
Resistance and
Cystic Fibrosis
Cystic Fibrosis
Highlight a Family of Membrane
Highlight a Family of Membrane
Proteins with ATP-Binding
Proteins with ATP-Binding
Cassette Domain
Cassette Domain s
s
Tumor cells in culture often
Tumor cells in culture often
become resistant to drugs that
become resistant to drugs that
were initially quite toxic to
were initially quite toxic to
the cells.
the cells.
The development of resistance
The development of resistance
to one drug also makes the
to one drug also makes the
cells less sensitive to a
cells less sensitive to a
range of other compounds. This
range of other compounds. This
phenomenon is known as
phenomenon is known as
multidrug resistance
multidrug resistance .
Good example is a membrane
Good example is a membrane
protein called the
protein called the multidrug
multidrug
resistance protein (MDR)
resistance protein (MDR) or
or P-
glycoprotein
glycoprotein (“glyco” because
(“glyco” because
it includes a carbohydrate
it includes a carbohydrate
moiety). Thus, when cells are
moiety). Thus, when cells are
cystic fibrosis
cystic fibrosis
chloride channel
chloride channel