

































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
An in-depth exploration of enzyme catalytic strategies, mechanisms, and inhibitors. Topics include transition states, activation energy, substrate binding, general acid base catalysis, competitive and noncompetitive inhibition, irreversible inhibitors, and active site mapping. Real-life examples like azt inhibition and penicillin are used to illustrate concepts.
Typology: Study notes
1 / 55

This page cannot be seen from the preview
Don't miss anything!
















































Tymoczko • Berg • Stryer
© 2010 W. H. Freeman and Company
In this lesson we will investigate how In this lesson we will investigate how Catalytic strategies of enzymes works Catalytic strategies of enzymes works and then how enzyme activity and then how enzyme activity
can be can be modulated by environmental factors modulated by environmental factors distinct from allosteric signals distinct from allosteric signals
All enzymatic reactions go through aAll enzymatic reactions go through a transition state (unstable intermediate form with a structure between that of reactant and product).transition state (unstable intermediate form with a structure between that of reactant and product).
Reactants must collide precisely to form transition state. Reactants must collide precisely to form transition state.
Must haveMust have correct orientation.correct orientation. Must collide with enough energy = activation energy =Must collide with enough energy = activation energy = GG
Enzymes work byEnzymes work by loweringlowering GG Substrates are correctly oriented.Substrates are correctly oriented. All increase probability of reaction.All increase probability of reaction.
Transition states are stabilized.Transition states are stabilized. Another key aspect of enzymeAnother key aspect of enzyme catalyiscatalyis – –binding energy.binding energy. The fact that binding energy isThe fact that binding energy is maximalmaximal when the enzyme binds to the transition state favors the formation of the transition state and thereby promotes catalysis.when the enzyme binds to the transition state favors the formation of the transition state and thereby promotes catalysis.
Chemical CatalysisChemical Catalysis
Active site of most enzymes is lined with hydrophobic amino acids.Active site of most enzymes is lined with hydrophobic amino acids.
There are a few polar a.a. which make up the catalytic center of the active site and can be ionized.There are a few polar a.a. which make up the catalytic center of the active site and can be ionized. Histidine (basic a.a.) is common.Histidine (basic a.a.) is common.
Aspartate and glutamate - negatively chargedAspartate and glutamate - negatively charged Lysine and arginine - positively charged; electrostatic binding can occurLysine and arginine - positively charged; electrostatic binding can occur
Enzymes that use this have a.a. side chains that can donate or accept electrons to substrate.Enzymes that use this have a.a. side chains that can donate or accept electrons to substrate.
Can accelerate a chemical reaction by a factor of 10-100.Can accelerate a chemical reaction by a factor of 10-100.
In general acid –base catalysts, a molecule other than water plays the role of a proton donor or acceptor.In general acid –base catalysts, a molecule other than water plays the role of a proton donor or acceptor.
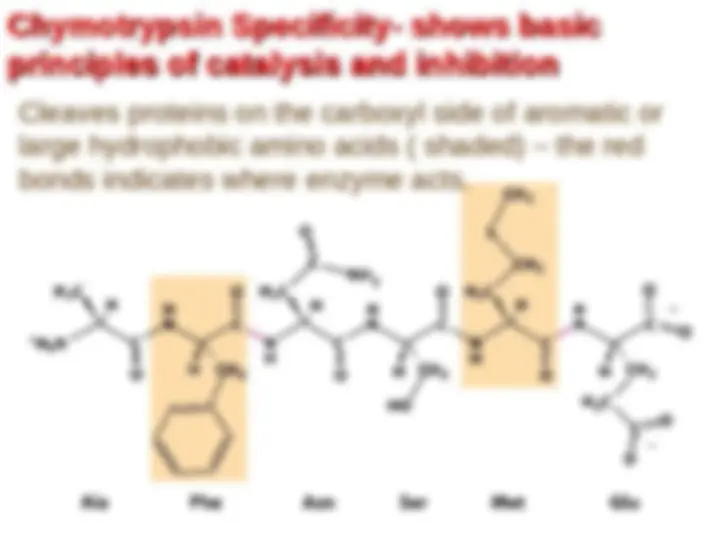
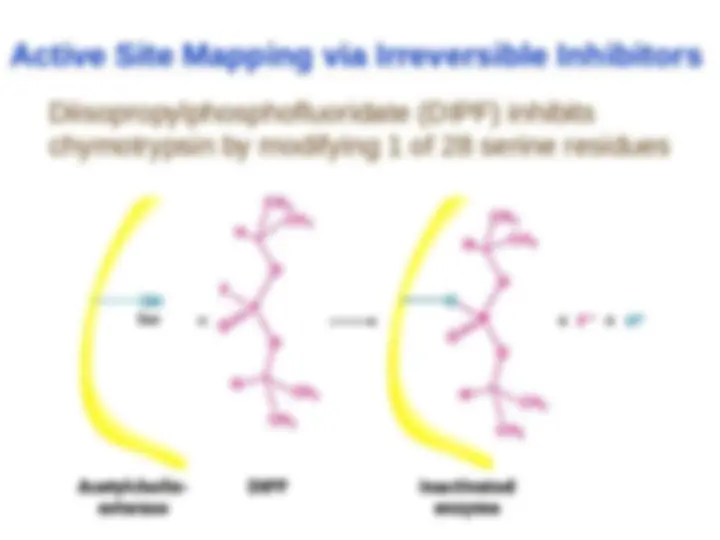
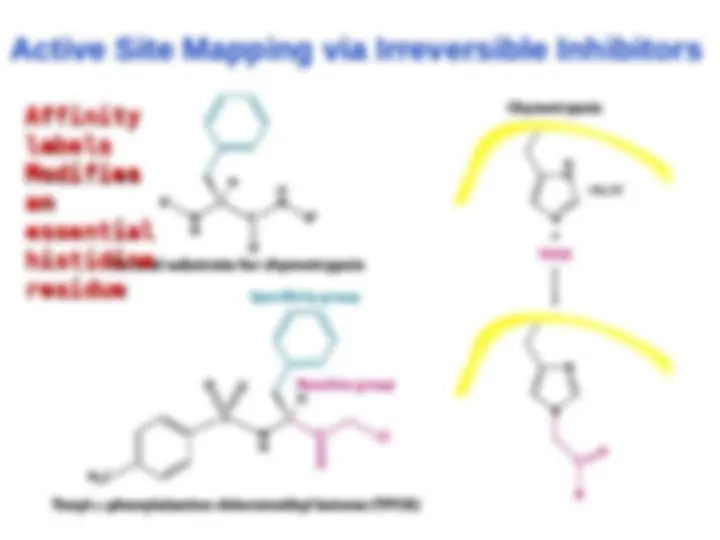
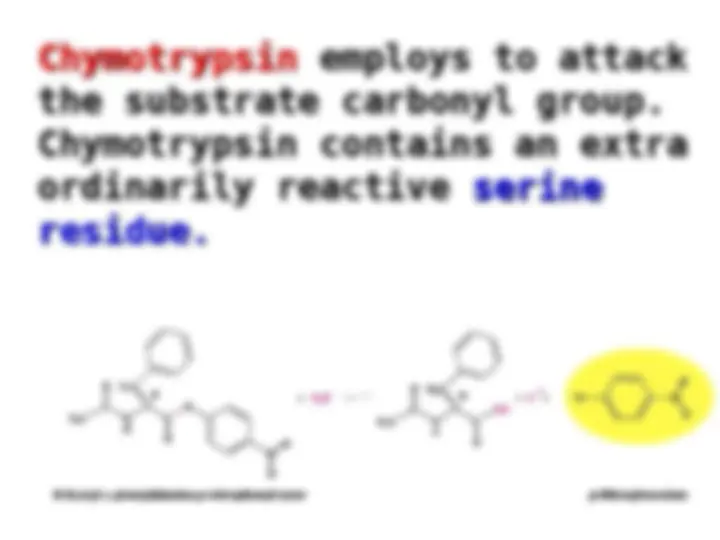
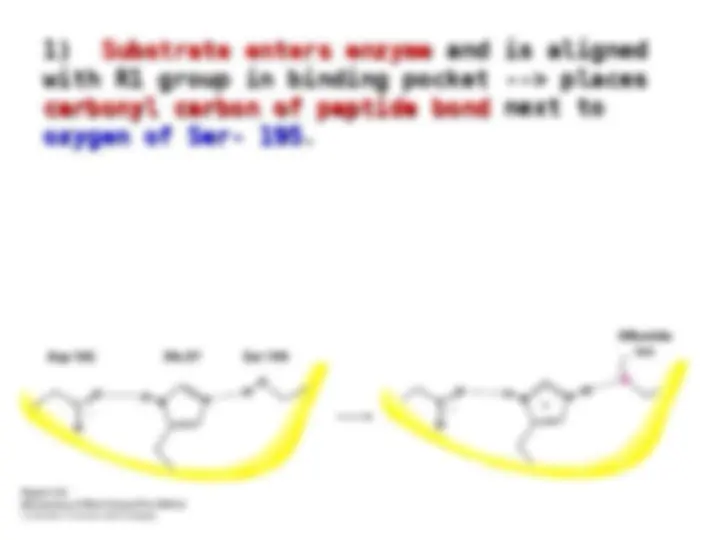
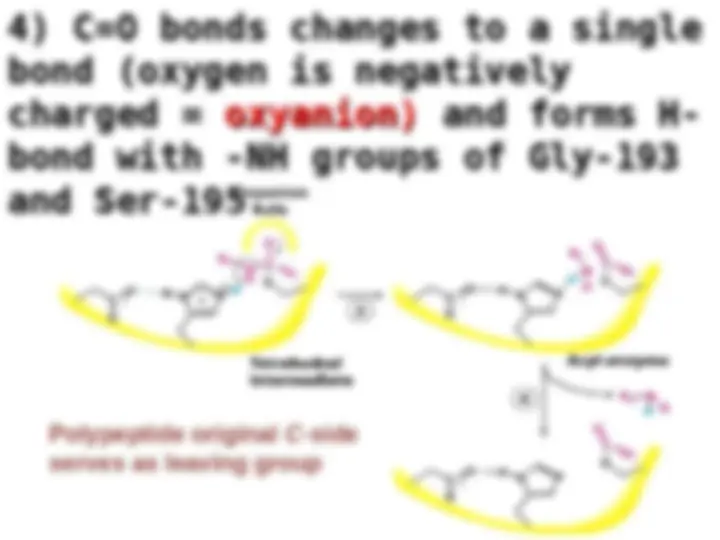
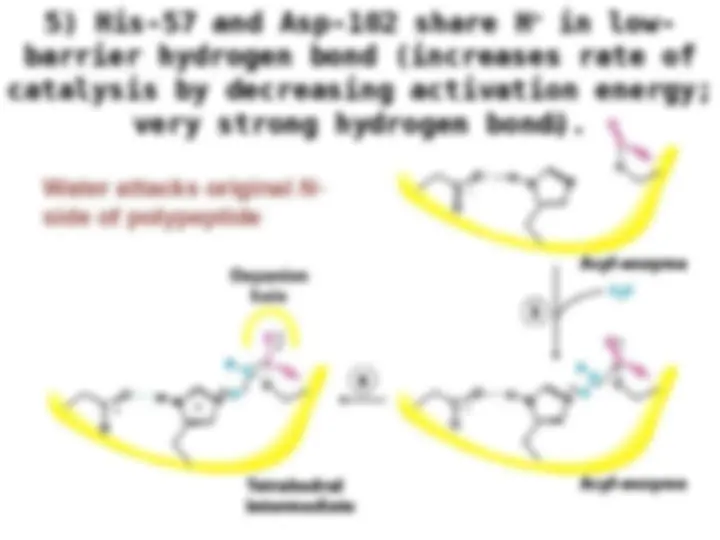
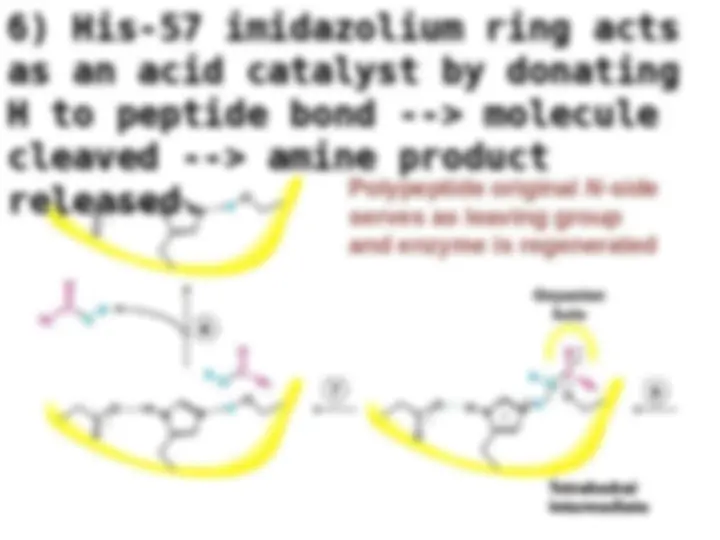
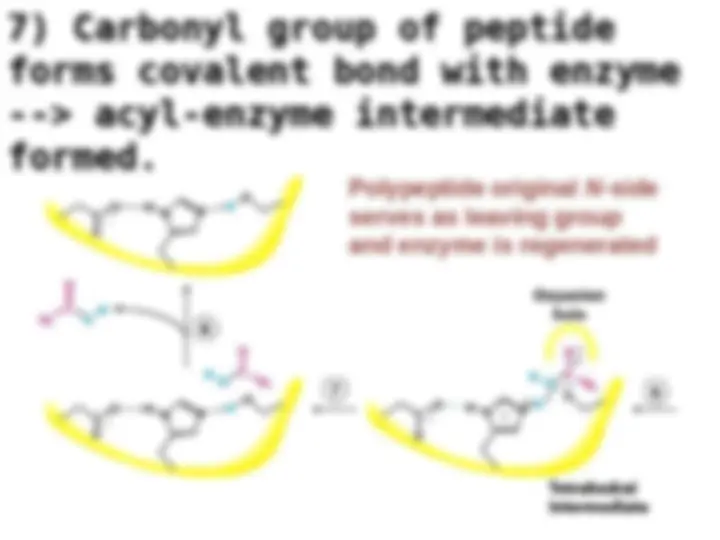
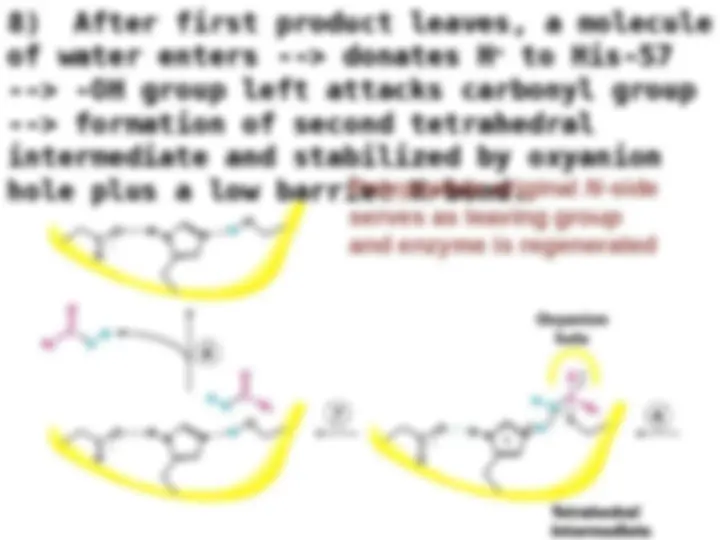
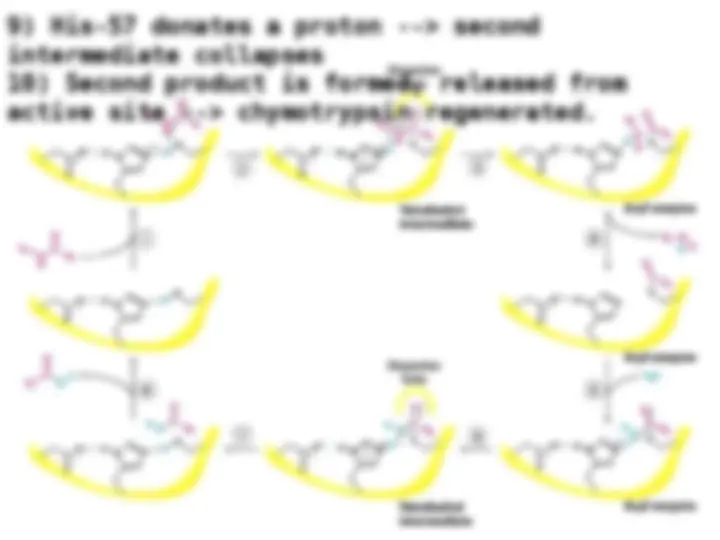
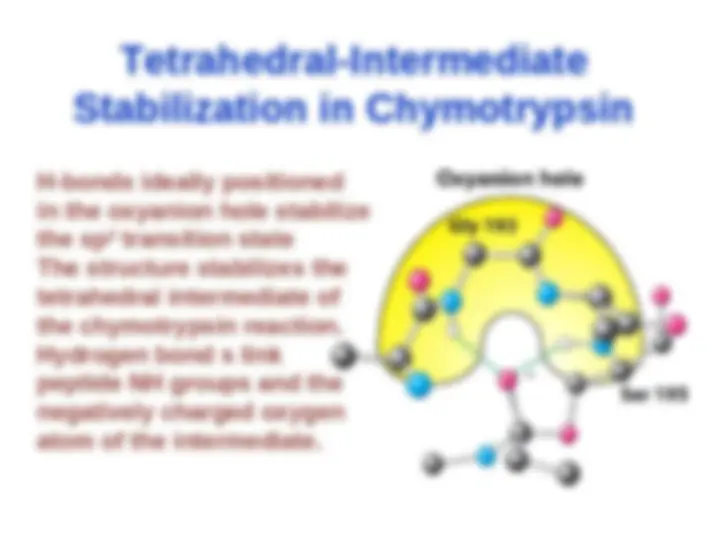
Chymotrypsin uses a histidine residue as a base catalyst to enhance the nucleophilic power of serine. Chymotrypsin uses a histidine residue as a base catalyst to enhance the nucleophilic power of serine.
Metal ion can function catalytically in several ways.Metal ion can function catalytically in several ways.
A.A.It serves as an electrophilic catalyst, stabilizing a negative charge on a reaction intermediate.It serves as an electrophilic catalyst, stabilizing a negative charge on a reaction intermediate.
B. B. Alternatively , the metal ion may generate a nucleophilic by increasing the acidity of a nearby molecule ,such as water. Alternatively , the metal ion may generate a nucleophilic by increasing the acidity of a nearby molecule ,such as water.
C.C.The metal ion may bind to the substrate , increasing the number of interactions with the enzyme and thus the binding energy.The metal ion may bind to the substrate , increasing the number of interactions with the enzyme and thus the binding energy.
Enzyme activity can be modulated by temperature , pH, and inhibitory molecules
Tyrosinase
part of the pathway that
synthesizes the pigment
that results in dark fur,
has low tolerance for
heat. It inactive at normal
temperature but
functional at lower
temperature.
Tyrosinase Activity Curve
Why are Siamese markings only on their
extremities? cool enough for tyrosine to gain
function and produce pigment
] Regulates Enzymatic Activity
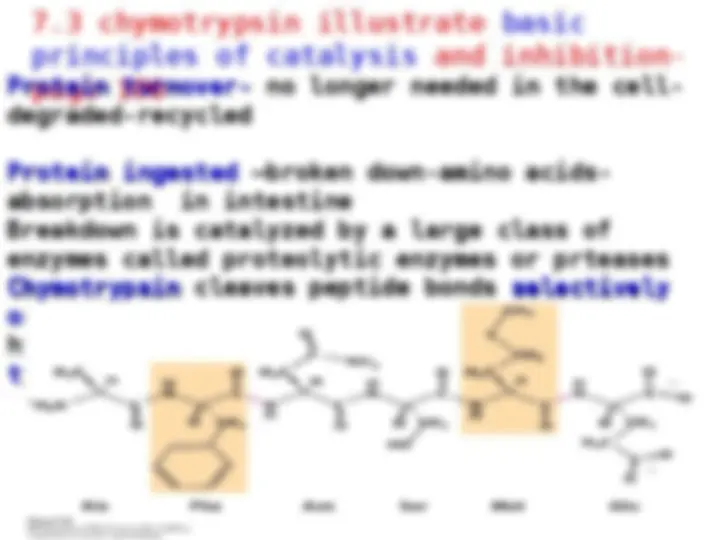
Protein Digestive Enzymes-
Pepsin stomach
Chymotrypsin
intestine
Activity of most
enzymes display a
bell shaped curve
Optimum
pH
varies
acid
ic
basic
If the pH is lowered , the - coo- If the pH is lowered , the - coo-
group will converted into –COOH so less group will converted into –COOH so less
enzyme activity enzyme activity
If the pH is raised the –NH3 + group If the pH is raised the –NH3 + group
loses an H+ to OH - , becoming a neutral loses an H+ to OH - , becoming a neutral
**- NH2 group , and the enzyme activity
diminished. diminished.
The pH dependence of enzymes is due to The pH dependence of enzymes is due to
the presence of ionizable R groups the presence of ionizable R groups
Graphical representation of competitive Graphical representation of competitive
inhibitors: inhibitors:
affects K affects K
m m
(increases K (increases K
m m
--> decreases --> decreases
affinity; need more substrate to reach half- affinity; need more substrate to reach half-
saturation of enzyme) saturation of enzyme)
maxmax
unaffected unaffected
Substrate can out Substrate can out
compete inhibitor → compete inhibitor → V
maxmax
unchanged since unchanged since V
max max
k k
2 2
As concentration of a As concentration of a
competitive inhibitor competitive inhibitor
increases, higher increases, higher
concentrations of substrate concentrations of substrate
are required to attain a are required to attain a
particular reaction particular reaction
velocity. The reaction velocity. The reaction
pathway suggests how pathway suggests how
sufficiently high sufficiently high
Reaction
Pathway
Competitive Enzyme Inhibition page 95
Reaction Pathway
max max
maxmax
22
TT
M M
MM
-1-
22
11
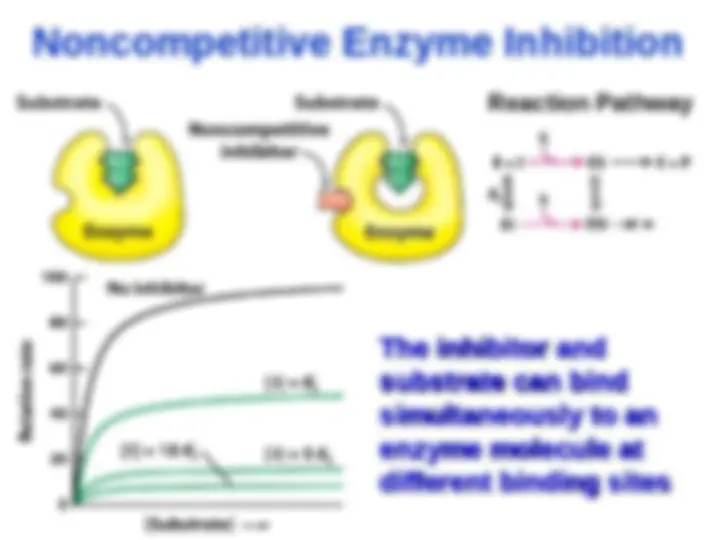
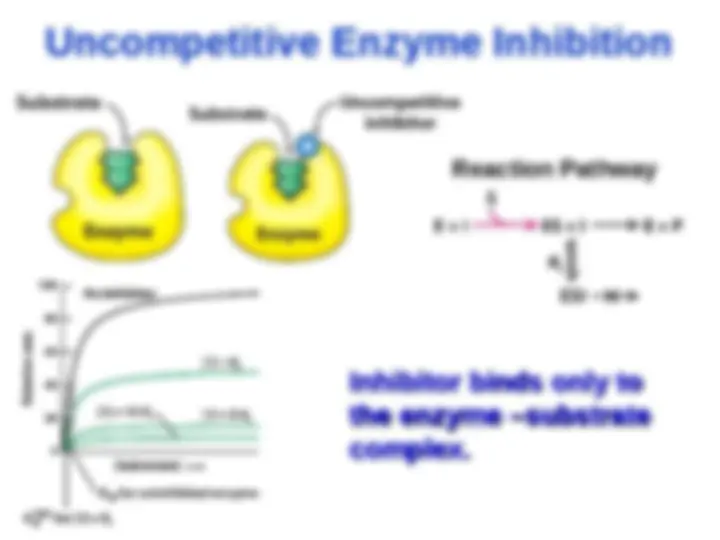
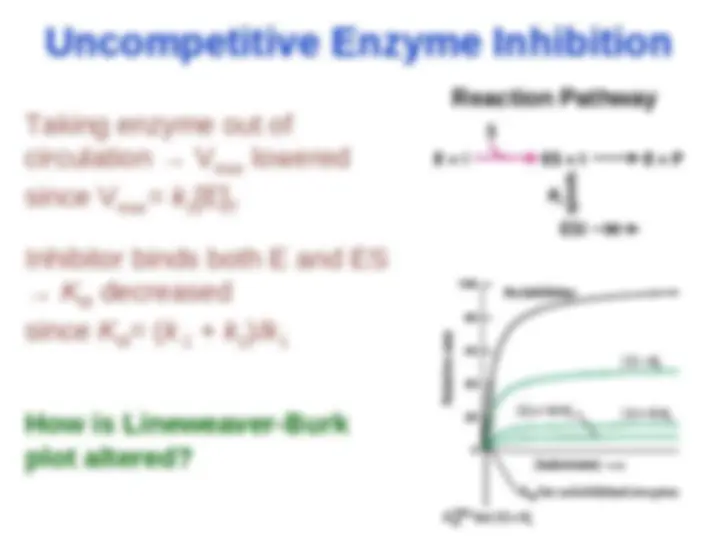
Noncompetitive Enzyme Inhibition
Reaction Pathway
Noncompetitive Enzyme Inhibition
Reaction Pathway
Taking Taking enzyme out of enzyme out of
circulation
circulation →
→ V
V
max max
lowered lowered since since V V
max max
k
k
2 2
[E]
[E]
Inhibitor binds both E Inhibitor binds both E
and ES
and ES →
→ K
K
M M
unchanged unchanged
since
since K
K
M M
= (
= ( k
k
k
k
2 2
)/
)/ k
k
1 1