





Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
An introduction to Lewis structures, focusing on covalent bonding and the systematic approach to drawing them. It covers the role of electronegativity, the process of creating octets and duplets, and the calculation of bonding types. Additional tips and examples are also included.
What you will learn
Typology: Study notes
1 / 8

This page cannot be seen from the preview
Don't miss anything!





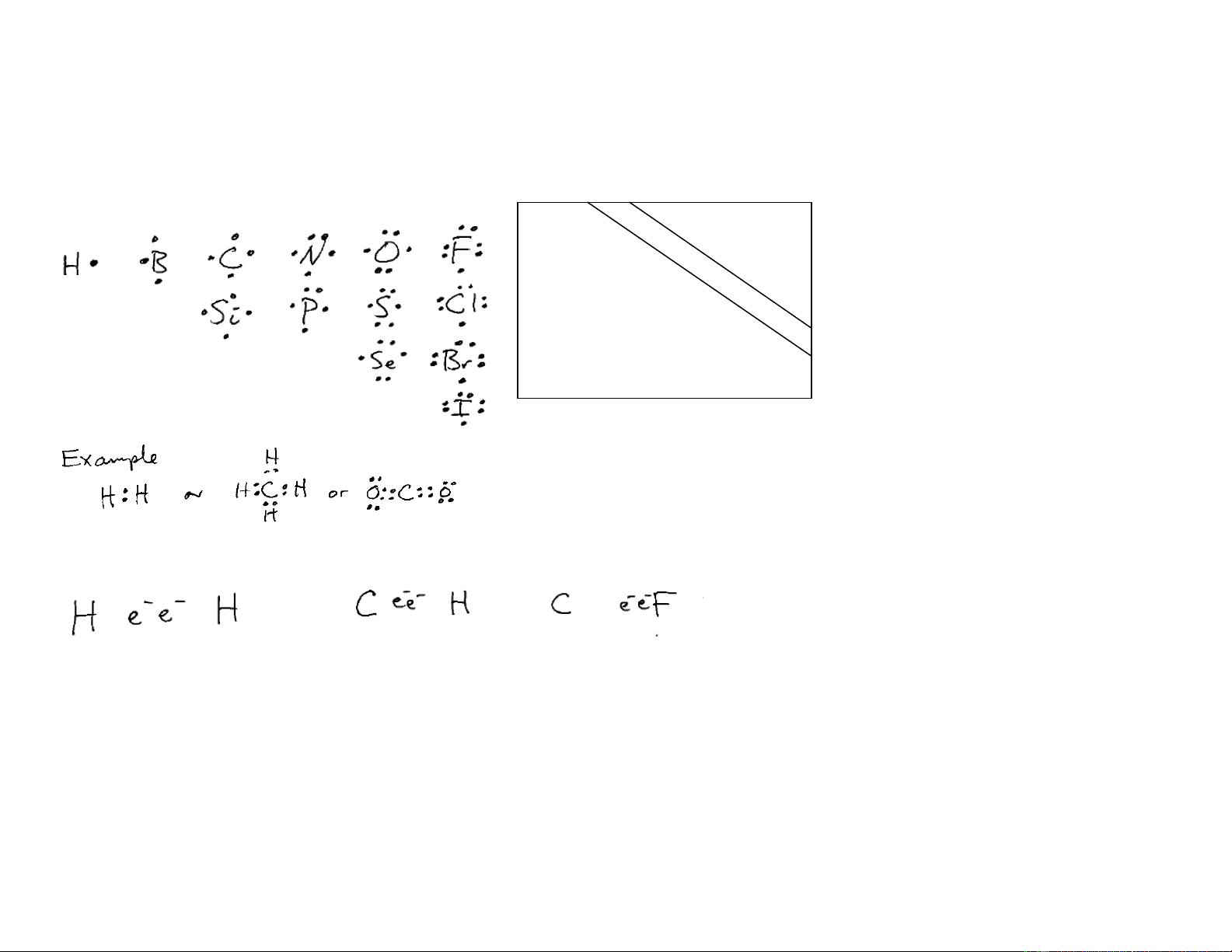
In the upper right corner of the periodic table are the non metals. These relatively few atoms have the following Lewisstructures. And we still want to mix andmatch to satisfy filled shellslike s2 + s2 p6 In all cases, covalent bonds involve the sharing of electrons. NO ions are formed. Note that even though the e¯ pair is shared, it is found closer to one atom than another. Electronegativity will explain this.
METALS
NON-
METALS
Systematic Approach to Drawing Lewis Structures
Is there a systematic way to build Lewis structures of molecules? Try this:
Write out the atoms symmetrically with the most electropositive in the center.
Count up all the valence electrons (H=1, C=4, N=5, etc)
NCl3=
2H
single bonds S= 40-32=8S/2/B=8/2/4= 1SingleBondsaround C
S/2/B=2doublebonds S=24-16=8S/2/B=8/2/2= 2doublebondsaround C
S/2/B= 3Triple bond S=16-10=6S/2/B=6/2/1=3triple
bond
between C andO
S/2/B=fractiongreater than 1 S=24-18=6S/2/B=
3/2 3 e¯ pairs sharedbetween
two
bonding regions
S/2/B<1 S=24-22S/2/B=2/1/2=1/2So
need
expanded
for
e¯ pairs
Explaining Resonance
Can you explain resonance? Sure, when we draw certain structures, there are equivalent ways to present them, when thishappens, we can draw multiple Lewis structures. The ones we see most often are when 3 e ¯ are placed in two e¯ regions
Note that when we get lazy, we draw
instead of all those different structures.
What about more examples of hypervalent compounds. (Ones with 5 or 6 e¯ rich regions)Sure. Just remember no to panic. The answer is either:
or
Example, PCl5= 40 e¯s: *Note: P must accommodate 5 bondsExample, XeF
4= 36 e¯s:
*Note after putting 32 e¯s
around perimeter F, we had 4 e¯s left over to add
to 6 e¯s rich regions, 4 bonding to Xe and 2
non-bonding.
Is the octet + duplet rule always followed?
Nope. There are 3 exceptions.
Too many electrons around the central atom, like SF
6, XeF
4, IBr
2¯, PCl
5, etc=happens with n=3 and higher situations with
d orbits available.
Too few electrons around the central atom like B or Be
Odd number of electrons in valence shells means you can’t form a
electron pair
empty orbit is an acid^ one e¯ is a radical (causes cancer) is a bond as a nonbonding e¯ pair (Lewis base)
Odd case. So if you ever count an odd number of total valence electrons, you have to form a radical.Example: Too few electrons means not enough to form octet.Example of Lewis atom structures with insufficient e¯s:
Too few electrons means you don’t have enough electrons to form four bonds. Hint: If you ever see a Boron compound, think octet rule exception.