




Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Material Type: Lab; Class: Gen College Chemistry I : SC1; Subject: Chemistry; University: Front Range Community College; Term: Spring 2008;
Typology: Lab Reports
1 / 6

This page cannot be seen from the preview
Don't miss anything!




Chemistry 111 – Aug 2008 5 - 1
A titration is a process used to determine the volume of a solution needed to react with a given amount of
another substance. In this experiment, you will titrate hydrochloric acid solution, HCl, with a basic sodium hydroxide solution, NaOH. The concentration of the NaOH solution is given and you will
determine the unknown concentration of the HCl. Hydrogen ions from the HCl react with hydroxide ions
from the NaOH in a one-to-one ratio to produce water in the overall reaction:
H+(aq) + Cl–(aq) + Na+(aq) +OH–(aq) → H 2 O(l) + Na+(aq) + Cl–(aq)
For a quantitative determination of the HCl concentration there must be a way to know when the titration
has reached the equivalence point. Because all of the reagents and products in this reaction are colorless,
an indicator will be added to signal when the titration has reached the equivalence point.
In this experiment we will simultaneously determine the equivalence point using an electronic
measurement of hydrogen ion concentration.
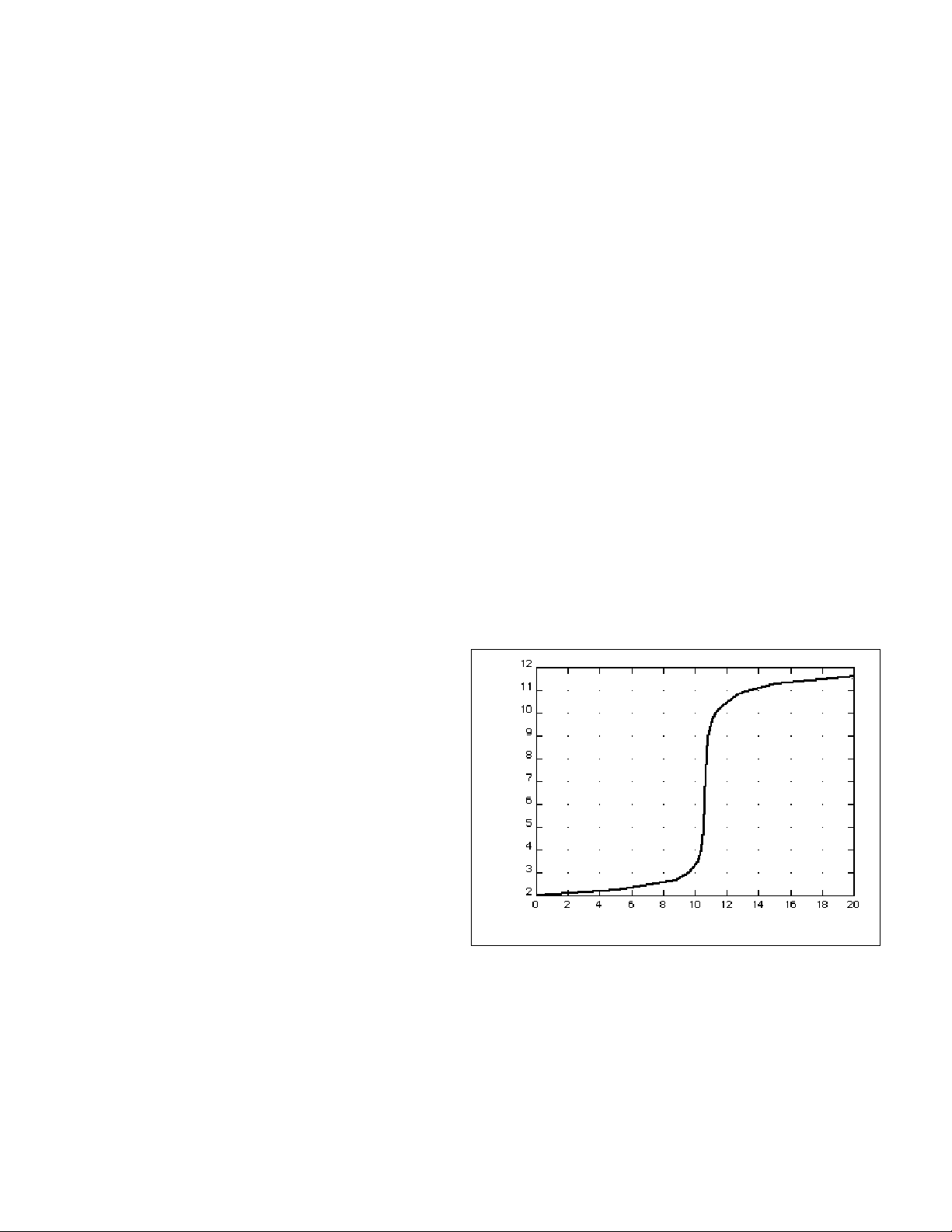
When an HCl solution is titrated with a NaOH solution, initially the acidity or concentration of acid is
very high. As base is added, the change in hydrogen ion concentration is quite gradual until close to the equivalence point, when equimolar amounts of acid and base have been mixed. Near the equivalence
point, the concentration of H+^ ions increases very rapidly, as shown in Figure 1. The change in hydrogen
ion concentration then becomes more gradual again, before leveling off with the addition of excess base. The equivalence point is indicated by the point at which the change in concentration is most rapid.
Note: Acidity or concentration of hydrogen ions
(written [H
]) is usually expressed as “pH”. The unit used for [H+] is pH = -log [H+]. (This is a
subject you will study in depth in CHE 112.)
For purposes of this experiment note that pH is inversely related to hydrogen ion concentration;
that is, as hydrogen ion concentration declines,
pH increases.
In this experiment, you will use a pH probe to
monitor H
ion concentration as you titrate and
the computer to record the data. The region of most rapid pH change will then be used to
determine the equivalence point. The volume of
NaOH titrant used at the equivalence point will be used to determine the molarity of the HCl.
Experiment 5 Chemistry 111
5 - 2 Chemistry 111
In this experiment, you will
Computer magnetic stirrer (if available) Vernier computer interface stirring bar or Microstirrer (if available) Logger Pro wash bottle Vernier pH Sensor distilled water HCl solution, unknown concentration ring stand 0.100 M NaOH solution 1 utility clamp pipet bulb (^) 2 x 250 mL beaker 25 mL buret 10 mL pipet
Experiment 5 Chemistry 111
5 - 4 Chemistry 111
Trial 1 Trial 2
Concentration of NaOH M M
NaOH volume added before the largest pH increase mL mL
NaOH volume added after the largest pH increase mL mL
Volume of NaOH added at equivalence point mL mL
Volume of NaOH added at end point (Where indicator changes color.)
mL mL
Moles NaOH
mol mol Moles HCl
mol mol Concentration of HCl
mol/L mol/L Average [HCl] M
An alternate way of determining the precise equivalence point of the titration is to take the first and second derivatives of the pH-volume data. The equivalence point volume corresponds to the peak (maximum) value of the first derivative plot, and to the volume where the second derivative equals zero on the second derivative plot.
View the first-derivative graph (∆pH/∆vol) by clicking the on the vertical-axis label (pH), and choose First Derivative. You may need to autoscale the new graph by clicking the Autoscale button,. View the second-derivative graph (∆ 2 pH/∆vol 2 ) by clicking on the vertical-axis label, and choosing Second Derivative.
Chemistry 111 Acid-Base Titration
Chemistry 111 5 - 5
POST-LAB NAME: ___________________________________
concentration for the unknown acid be too high or too low?
a. One drop of NaOH solution hit the side of the beaker and spilled out.
b. When using the pipette to introducing 10 mL of HCl solution, you blew out the last bit of solution.
c. You failed to wash down the sides of the beaker so drops of solution remained on the sidewalls.
end point? Would your result be affected by use of the end point value? Calculate the percent error introduced by this difference.
required to reach the equivalence point, what is the concentration of acid in the unknown?