



















Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Overview. • Some general observations. • The “prime-time-players”…what were ... 13C spectroscopy a new thing in organic chemistry?!? ... David Evans (UCLA).
Typology: Lecture notes
1 / 26

This page cannot be seen from the preview
Don't miss anything!



















E.J. Corey (Harvard)
CO
2
H Me
Me
P
Ph
Ph
Hunig's Base
C
5
H
11
Hexanal
O
O
CO
2
Me
Me
C
5
H
11
Generally, E/Z > 1
90 - 92 %
9 - 10 %
E. J. Corey (p. 256)
Samuel Danishefsky (Univ. of Pitt)
2
2
2
2
2
2
2
2
2
Gilbert Stork (Columbia)
Barry M. Trost (Wisconson, Madison)
Alkylations
NaH
CH
3
SO
2
CH
2
CO
2
CH
3
1 , 2 , or 3
H
3
CO
2
S CO
2
CH
3
LiI, NaCN, DMF
130 °C
H
3
CO
2
S
2
CH
2
OH,
TsOH, PhH, Reflux
2
H
5
NH
2
, 0 °C
3 .H
2
O, HCl
Barry M. Trost (Wisconson, Madison)
N
N N
O
O
Li
O
Me
Me
Me
LDA, THF
N
N N
OMe OMe
Li
LDA, THF
E
E
OMe
E
E
1
2
chlorosilanes.
for 2 , (weaker reagent), reaction to MVK proceeded with conjugate addition
This selectivity is due to the less reactive reagent having its charge more delocalized in the
transition state. To test this hypothesis, a controll exmeriment:
MVK
22 hr,
RT
N OMe
Me
N O
Me
O
Exclusively 1 , 4 addition, 29 %
R. F. Heck (Univ. of Delaware :-) )
2
'
3
2
3
2
R. F. Heck (Univ. of Delaware :-) )
Br
2
3
Pd(Br)
2
(PPh
3
2
2
1
Possible Mechanism:
(S)-Carlosic Acid
Blommer and Kappler, p. 113
Temple University
MeO
2
t-BuOK
t-BuOH
2
Me
MeO
2
Br
2
AcOH
MeO
2
Br
OMe
MeO
Et
3
N (cat), PhH
2
, Pd/C
AcOH
MeO
2
Cl
TiCl
4
PhNO
2
MeO
2
HCl
NaOH
2
(S)-Carlosic Acid
Note: all yields in the synthesis were 70 - 80 %, except the "key step," the cyclization
O
O
OMe
NH
2
O
O
OH
O
Br
Known Compounds
O
O
OMe
H
N O
Br
O
O
PhH, 2 hr
76 %
O
O
OMe
N
O O
Br
POCl
3
CH
3
CN
20 hr, 90 %
O
O
OMe
N
O O
Br O
Ethyl OEt
Chloroformate
Et
3
N, 0 °C
90 %
O
O
OMe
N
O
O
O
OEt
t-BuOK
hn, 7 hr
PhH, t-BuOH
32 %
O
O
OMe
N
O
O
Me
LiAlH
4
, AlCl
3
Et
2
O, reflux
O
O
OMe
N
O
O
Me
O
O
O
H
AcOH
23 %
Cassamedine
OAc
2
, Pd/BaSO
4
Pentane, 84 %
OAc
NaNO
2 (aq)
3 (aq)
OAc
2
, Pd/BaSO
4
Pentane, 80 %
OAc
The Sex Pheramone of the
Pink Bollworm
The Sex Pheromone of the Pink Bollworm
Phillip E. Sonnet, p. 3793
U.S. Dept. of Agriculture, Maryland
A Trail Pheromone of the Pharaoh Ant
Sonnet, Oliver, p. 2663
U.S. Dept. of Agriculture, Maryland
methoctahydroindolizine, but absolute stereochemistry of active pheromone was not
elucidated
pheromone's utility as a pest control agent
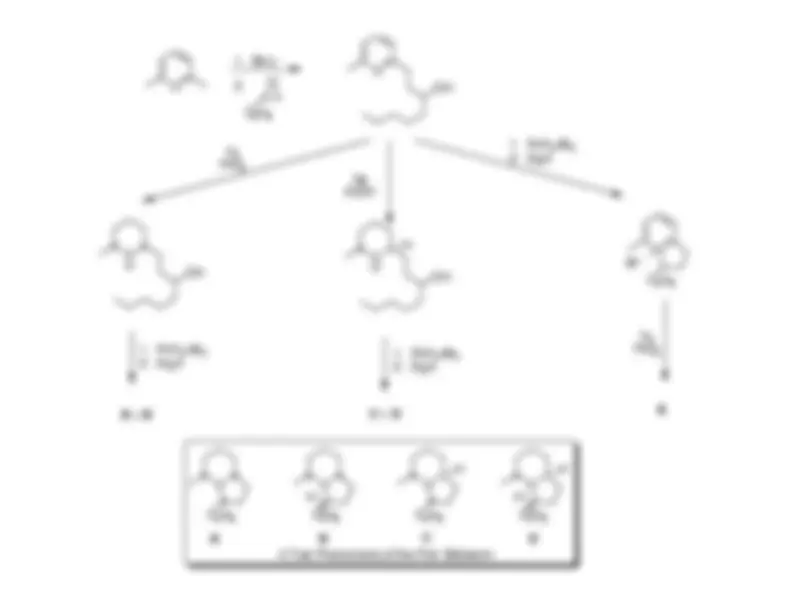
N
C
H
N
C
H
N
C
H
N
C
H
H
H
H
H
A
B
C D
A Trail Pheromone of the Pink Bollworm