










Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Pharmacology is the study of the actions, mechanisms, uses and adverse effects of drugs. A drug is any natural or synthetic substance that alters.
Typology: Lecture notes
1 / 17

This page cannot be seen from the preview
Don't miss anything!










What is pharmacology?
Pharmacology is the study of the actions, mechanisms, uses
and adverse effects of drugs.
A drug is any natural or synthetic substance that alters
the physiological state of a living organism. Drugs can be
divided into two groups.
treatment and diagnosis of disease.
recreational purposes. These drugs include illegal
substances such as cannabis, heroin and cocaine, as
well as everyday substances such as caffeine, nicotine
and alcohol (see Chapter 9).
Although drugs may have a selective action, there is always
a risk of adverse effects associated with the use of any drug,
and the prescriber should assess the balance of desired and
adverse effects when deciding which drug to prescribe.
Drug names and classification
A single drug can have a variety of names and belong to
many classes. Drugs are classified according to their:
When a drug company's patent expires, the marketing of
the drug is open to other manufacturers. Although the ge-
neric name is retained, the brand names can be changed.
How do drugs work?
Most drugs produce their effects by targeting specific cel-
lular macromolecules, often proteins. The majority act as
receptors in cell membranes, but they can also inhibit en-
zymes and transporter molecules. Some drugs directly
interact with molecular targets found in pathogens. For ex-
ample, β-lactam antibiotics are bactericidal, acting by inter-
fering with bacterial cell wall synthesis.
Certain drugs do not have conventional targets. For
example, succimer is a chelating drug that is used to treat
heavy metal poisoning. It binds to metals, rendering them
inactive and more readily excretable. Such drugs work by
means of their physicochemical properties and are said to
have a nonspecific mechanism of action. For this reason,
these drugs must be given in much higher doses than the
more specific drugs. Another example would be antacids
used to reduce the effect of excessive acid secretion in the
stomach.
Transport systems
Ion channels are proteins that form pores in the cell mem-
brane and allow selective transfer of ions (charged species)
in and out of the cell. Opening or closing of these channels
is known as gating; this occurs as a result of the ion channel
undergoing a change in shape. Gating is controlled either by
a neurotransmitter (receptor operated channels) or by the
membrane potential (voltage-operated channels).
Some drugs modulate ion channel function directly by
blocking the pore (e.g. the blocking action of local anaes-
thetics on sodium channels); others bind to a part of the ion
channel protein to modify its action (e.g. anxiolytics acting
on the γ-aminobutyric acid [GABA] channel). Other drugs
interact with ion channels indirectly via a G-protein and
other intermediates.
Carrier molecules located in the cell membrane facilitate
the transfer of ions and molecules against their concentra-
tion gradients. There are two types of carrier molecule.
(move one type of ion/molecule in one direction),
symporters (move two or more ions/molecules) or
antiporters (exchange one or more ions/molecules for
one or more other ions/molecules).
pumps (e.g. the Na
/K
adenosine triphosphatase
[ATPase] pump).
Enzymes
Enzymes are protein catalysts that increase the rate of spe-
cific chemical reactions without undergoing any net change
themselves during the reaction. All enzymes are potential
targets for drugs. Drugs either act as a false substrate for the
enzyme or inhibit the enzyme's activity directly, usually by
binding the catalytic site on the enzyme (Fig. 1.1).
Certain drugs may require enzymatic modification. This
degradation converts a drug from its inactive form (prod-
rug) to its active form.
Receptor
type
Time for
effect
Receptor
example
Function
example
Ion
channel–
linked
Milliseconds Nicotinic
acetylcholine
receptor
Removing
hand from
hot water
G-protein–
linked
Seconds β-Adrenergic
receptor
Airway
smooth
muscle
relaxation
Tyrosine
kinase–
linked
Minutes Insulin
receptor
Glucose
uptake into
cells
DNA-linked Hours to
days
Steroid
receptor
Cellular
proliferation
Receptors Table 1.1 The four main types of receptor and their uses
Receptors are the means through which endogenous ligands
produce their effects on cells. A receptor is a specific protein
molecule usually located in the cell membrane, although in-
tracellular receptors and intranuclear receptors also exist.
A ligand that binds and activates a receptor is an agonist.
However, a ligand that binds to a receptor but does not ac-
tivate the receptor and prevents an agonist from doing so is
called an antagonist.
The following are naturally occurring ligands.
terminals that diffuse across the synaptic cleft, and bind
to presynaptic or postsynaptic receptors.
or into the bloodstream from specialized cells, can act
at neighbouring or distant cells.
Each cell expresses only certain receptors, depending on the
function of the cell. Receptor number and responsiveness to
external ligands can be modulated.
In many cases, there is more than one receptor for each
messenger so that the messenger often has different phar-
macological specificity and different functions according to
where it binds (e.g. adrenaline is able to produce different
effects in different tissues because different adrenergic re-
ceptors are formed of different cell types).
There are four main types of receptor (Table 1.1).
Receptors that are directly linked to ion channels (Fig. 1.2)
are mainly involved in fast synaptic neurotransmission. A
classic example of a receptor linked directly to an ion chan-
nel is the nicotinic acetylcholine receptor (nicAChR).
The nicAChRs possess several characteristics:
both α subunits to activate the receptor.
Ions Ions
R R
Hyperpolarization
or
depolarization
Charge
inexcitability
Ca
2+ release
Protein
phosphorylation
Other
or
Second messengers
Cellular effects Cellular effects Cellular effects Cellular effects
Protein synthesis Protein synthesis
Gene transcription
Nucleus
Gene
transcription
Protein
phosphorylation
R
R
E R/E
G G
1. Ligand-gated ion
channels (ionic receptors)
2. G protein-coupled
receptors (metabotropic)
3. Kinase-linked 4. Nuclear receptors
receptors
Time scale
Milliseconds
Examples
Nicotinic
ACh receptor
Seconds
Muscarinic
ACh receptor
Hours
Estrogen receptor
Hours
Cytokine receptors
+ − + or −
Fig. 1.1 How ion channel enzymes work. ACh, acetylcholine. (From Rang HP, Dale MM, Ritter JM, Moore PK.
Pharmacology. 8
th ed. Edinburgh: Churchill Livingstone, 2016.)
Enzymes, transport
proteins, etc.
Contractile
proteins
Ion
channels
Target
enzymes
Second
messengers
Protein
kinases
Effectors
Released
as local
hormones
PKA PKG PKC
Eicosanoids
cAMP cGMP IP DAG AA 3
Ca
2
Adenylyl
cyclase
Guanylyl
cyclase
G-protein
Phospholipase C
Fig. 1.5 Second-messenger targets of G-proteins and their effects. AA, arachidonic acid; cAMP, cyclic adenosine
monophosphate; cGMP, cyclic guanosine monophosphate; DAG, diacylglycerol; IP 3 , inositol (1,4,5) triphosphate; PK,
protein kinase.
Guanosine triphosphate (GTP) replaces GDP in the
cleft thereby activating the G-protein and causing the α
subunit to dissociate from the βγ dimer (see Fig. 1.4B).
G-protein (although this is not always the case: in
the heart, potassium channels are activated by the
βγ dimer and recent research has shown that the γ
subunit alone may play a role in activation). This
component diffuses in the plane of the membrane
where it is free to interact with downstream effectors
such as enzymes and ion channels. The βγ dimer
remains associated with the membrane owing to its
hydrophobicity (see Fig. 1.4C).
enzymic activity, hydrolyses the bound GTP to GDP.
The GDP-bound α subunit dissociates from the effector
and recombines with the βγ dimer (see Fig. 1.4D).
This whole process results in an amplification effect because
the binding of an agonist to the receptor can cause the ac-
tivation of numerous G-proteins, which in turn can each,
via their association with the effector, produce many other
molecules intracellularly.
Many types of G-protein exist. This is probably attrib-
utable to the variability of the α subunit. G s and G i
o
cause stimulation and inhibition, respectively, of the target
enzyme adenylyl cyclase. This explains why muscarinic
ACh receptors (G i
o
–linked) and β-adrenoreceptors (G s
linked) located in the heart produce opposite effects. The
bacterial toxins cholera and pertussis can be used to deter-
mine which G-protein is involved in a particular situation.
Each has enzymic action on a conjugation reaction with the
α subunit, such that:
adenylyl cyclase. This explains why infection with
cholera toxin results in uncontrolled fluid secretion
from the gastrointestinal tract.
of adenylyl cyclase. This explains why infection with
Bordetella pertussis causes a “whooping” cough,
characteristic of this infection, because the airways are
constricted, and the larynx experiences muscular spasms.
G-proteins interact with either ion channels or secondary
messengers. G-proteins may activate ion channels directly,
for example, muscarinic receptors in the heart are linked to
potassium channels which open directly on interaction with
the G-protein, causing a slowing down of the heart rate.
Secondary messengers are a family of mediating chemicals
that transduces the receptor activation into a cellular response.
These mediators can be targeted, and three main secondary
messenger systems exist as targets of G-proteins (Fig. 1.5).
Drug–receptor interactions 11
Adenylyl cyclase/cyclic adenosine monophosphate
system— Adenylyl cyclase catalyses the conversion of ATP
to cyclic adenosine monophosphate (cAMP) within cells.
The cAMP produced causes activation of certain protein
kinases, enzymes that phosphorylate serine and threonine
amino acid residues in various proteins, thereby producing
either activation or inactivation of these proteins. An ex-
ample of this system can be observed in the activation of
β 1
vation of β 1
cAMP- dependent protein kinase A, which phosphorylates
and opens voltage-operated calcium channels. This in-
creases calcium levels in the cells and results in an increased
rate and force of contraction. An inhibitory example of this
system can be observed in activation of opioid receptors.
The receptor linked to the “G i
” protein inhibits adenylyl cy-
clase and reduces cAMP production.
Phospholipase C/inositol phosphate system— Activation
of M 1
3
, 5-hydroxytryptamine (5-HT 2
), peptide and α 1
adrenoreceptors, via G q
, cause activation of phospholipase
C, a membrane-bound enzyme, which increases the rate of
degradation of phosphatidylinositol (4,5) bisphosphate into
diacylglycerol (DAG) and inositol (1,4,5) triphosphate (IP 3
DAG and IP 3
act as second messengers. IP 3
binds to the
membrane of the endoplasmic reticulum, opening calcium
channels and increasing the concentration of calcium within
the cell. Increased calcium levels may result in smooth mus-
cle contraction, increased secretion from exocrine glands,
increased hormone or transmitter release, or increased force
and rate of contraction of the heart. DAG, which remains
associated with the membrane owing to its hydrophobic-
ity, causes protein kinase C to move from the cytosol to the
membrane where DAG can regulate the activity of the latter.
There are at least six types of protein kinase C, with over
50 targets which can lead to:
Guanylyl cyclase system— Guanylyl cyclase catalyses
the conversion of GTP to cyclic guanosine monophosphate
(cGMP). This cGMP goes on to cause activation of protein
kinase G which in turn phosphorylates contractile proteins
and ion channels. Transmembrane guanylyl cyclase activ-
ity is exhibited by the atrial natriuretic peptide receptor
upon the binding of atrial natriuretic peptide. Cytoplasmic
guanylyl cyclase activity is exhibited when bradykinin acti-
vates receptors on the membrane of endothelial cells to gen-
erate nitric oxide, which then acts as a second messenger to
activate guanylyl cyclase within the cell.
Tyrosine kinase-linked receptors are involved in the regula-
tion of growth and differentiation, and responses to metabolic
signals. The response time of enzyme-initiated transduction
is slow (minutes). Examples include the receptors for insulin,
platelet-derived growth factor and epidermal growth factor.
Activation of tyrosine kinase receptors results in auto-
phosphorylation of tyrosine residues leading to the activa-
tion of pathways involving protein kinases. These receptors
have become important targets for certain types of antican-
cer drugs (see Chapter 13).
Deoxyribonucleic acid (DNA)–linked receptors are located
intracellularly and so agonists must pass through the cell
membrane to reach the receptor. The agonist binds to the
receptor and this receptor–agonist complex is transported
to the nucleus, aided by chaperone proteins. Once in the
nucleus, the complex can bind to specific DNA sequences
and so alter the expression of specific genes. As a result,
transcription of this specific gene to messenger ribonu-
cleic acid (mRNA) is increased or decreased and thus the
amount of mRNA available, for translation into a protein,
increases or decreases. The process is much slower than for
other receptor–ligand interactions, and the effects usually
last longer. Examples of molecules with DNA-linked recep-
tors are corticosteroids, thyroid hormone, retinoic acid and
vitamin D.
Most drugs produce their effects by acting on specific pro-
tein molecules called receptors.
Receptors respond to endogenous chemicals in the body
that are either synaptic transmitter substances (e.g. ACh,
noradrenaline) or hormones (endocrine, e.g. insulin; or lo-
cal mediators, e.g. histamine). These chemicals or drugs are
classed in two ways.
response.
cause activation. Antagonists reduce the chance of
transmitters or agonists binding to the receptor and
thereby oppose their action by effectively diluting or
removing the receptors from the system.
Electrostatic forces initially attract a drug to a recep-
tor. If the shape of the drug corresponds to that of the
Drugs, like naturally occurring chemical mediators,
act on receptors located in the cell membrane, in
the cytoplasm of the cell, or in the cell nucleus,
to bring about a cellular, and eventually organ or
tissue, response.
Drug–receptor interactions 11
produced by the antagonist reflects the affinity of the
antagonist for the receptor. High-affinity antagonists
stay bound to the receptor for a relatively long period
of time allowing the agonist little chance to take the
antagonist's place.
This concept can be quantified in terms of the dose ratio
(known as a Schild plot). The dose ratio is the ratio of the
concentration of agonist producing a given response in the
presence and absence of a certain concentration of antago-
nist, for example, a dose ratio of 3 tells us that three times
as much agonist was required to produce a given response
in the presence of the antagonist than it did in its absence.
Noncompetitive antagonists are also known as irreversible
antagonists.
shift to the right of the agonist dose–response curve
(see Fig. 1.7).
reflecting the fact that the antagonist's effect cannot be
overcome by the addition of greater doses of agonist. At
low concentrations, however, a parallel shift may occur
without a reduced maximum response. This tells us
that not all the receptors need to be occupied to elicit
a maximum response because irreversible antagonists
effectively remove receptors, there must be a number of
spare receptors.
Although on a log scale the relation between the concen-
tration of agonist and the response produces a symmetric
sigmoid curve, rarely does a 50% response correspond to 50%
receptor occupancy. This is because there are spare receptors.
This excess of receptors is known as receptor reserve and
serves to sharpen the sensitivity of the cell to small changes in
agonist concentration. The low efficacy of partial agonists can
be overcome in tissues with a large receptor reserve and in
these circumstances, partial agonists may act as full agonists.
Potency relates to the concentration of a drug needed to
elicit a response. The EC 50 , where EC stands for effective
concentration, is a number used to quantify potency. EC 50
is the concentration of drug required to produce 50% of
the maximum response. Thus the lower the EC 50 , the more
potent the drug. For agonists, potency is related to both af-
finity and efficacy, but for antagonists, only affinity is con-
sidered because they have no efficacy (Table 1.2).
Other variables can affect the efficacy of a drug beyond
its potency. For example, if a potent drug in vitro is metab-
olized in the stomach or affected by the pH in the stomach,
less would be available to reach the target site. This means
that, if given as a tablet, it would be less than the in vitro
potency predicted.
the biological effect of the drug on the body) is
influenced by many factors which are covered by the
term pharmacokinetics: the way the body affects the
drug with time, that is, the factors that determine its
absorption, distribution, metabolism and excretion.
A 22-year-old man is admitted to hospital with
signs of respiratory depression, drowsiness,
bradycardia and confusion. His girlfriend tells the
medical team that he uses heroin and an overdose
is therefore suspected. Heroin acts as an agonist,
activating the opioid receptors. Naloxone is a
competitive antagonist at those receptors and so is
administered as treatment. Minutes later the man's
condition improves, and his respiratory rate returns
to normal. Careful titration of the naloxone dose
should allow treatment of respiratory depression
without provoking acute withdrawal signs.
Log agonist concentration
Tissue response
Agonist alone
Agonist and
competitive antagonist
Agonist and irreversible
antagonist (low dose)
Agonist and irreversible
antagonist (high dose)
Fig. 1.7 Comparison of the log dose–response curves for
competitive and noncompetitive (irreversible) antagonists.
(From Neal MJ. Medical Pharmacology at a Glance, 6th
edition. Wiley-Blackwell, 2009.)
Definition Explanation
Affinity Number of bonds and goodness of
fit between drug and receptor.
Agonist A ligand that binds and activates a
receptor.
Antagonist A ligand that binds to but does not
activate a receptor. Prevents an
agonist from binding.
Efficacy The ability of agonists, once bound,
to activate receptors.
Potency Concentration of a drug needed to
elicit a response.
Table 1.2 Key definitions
Pharmacology can be divided into two disciplines. These are:
Pharmacokinetics and Pharmacodynamics
Administration
The drug can be administered by a variety of routes.
Topical drugs are applied where they are needed, giving them
the advantage that they do not have to cross any barriers or
membranes. This means a higher concentration of the drug
in the target tissue, with less drug being absorbed into the sys-
temic circulations and therefore less likelihood of unwanted
side effects. Examples include skin ointments; ear, nose or eye
drops; and aerosols inhaled in the treatment of asthma.
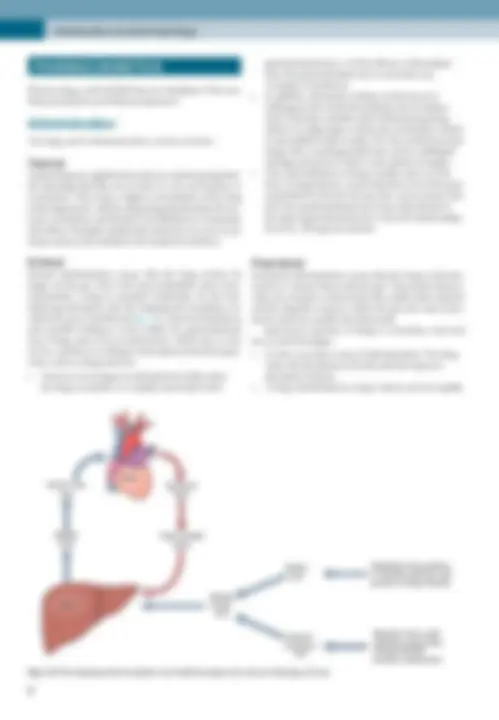
Enteral administration means that the drug reaches its
target via the gut. This is the least predictable route of ad-
ministration, owing to potential metabolism by the liver
following absorption into the hepatoportal circulation (so
called first pass metabolism)(Fig. 1.8), chemical breakdown
and possible binding to food within the gastrointestinal
tract. Drugs must cross several barriers, which may or may
not be a problem according to their physicochemical prop-
erties, such as charge and size.
the drug is unstable, or is rapidly inactivated in the
gastrointestinal tract, or if the efficacy of absorption
from the gastrointestinal tract is uncertain (e.g.
vomiting or diarrhoea).
sublingual route avoids the hepatoportal circulation
and is, therefore valuable when administering drugs
subject to a high degree of first-pass metabolism (which
is unavoidable if taken orally). It is also useful for potent
drugs with a nondisagreeable taste, such as sublingual
nitroglycerin given to relieve acute attacks of angina.
form of suppositories, means that there is less first-pass
metabolism by the liver because the venous return from
the lower gastrointestinal tract is less than that from
the upper gastrointestinal tract. It has the disadvantage,
however, of being inconsistent.
Parenteral administration means that the drug is adminis-
tered in a manner that avoids the gut. The protein drug in-
sulin, for example, is destroyed by the acidity of the stomach
and the digestive enzymes within the gut and must, there-
fore be injected, usually subcutaneously.
Intravenous injection of drugs is sometimes used and
has several advantages.
enters the bloodstream directly and thus bypasses
absorption barriers.
Liver
Hepatic
veins
Inferior vena
cava
Heart
Abnormal
aorta
Proper hepatic
artery
Hepatic
portal
vein
Splenic
vein
Tributarires from portions
of stomach, pancreas, and
portions of large intestine
Superior
mesenteric
vein
Tributaries from small
intestines and portions
of large intestine,
stomach, and pancreas
Fig. 1.8 The hepatoportal circulation and arterial supply and venous drainage of liver.
d values that amount to less than a certain body
compartment volume indicate that the drug is
contained within that compartment. For example,
when the volume of distribution is less than 5 L, it is
likely that the drug is restricted to the vasculature.
d values less than 15 L implies that the drug is
restricted to the extracellular fluid.
d values greater than 15 L suggests distribution
within the total body water. Some drugs (usually
basic) have a volume of distribution that exceeds
body weight, in which case tissue binding is
occurring. These drugs tend to be contained
outside the circulation and may accumulate in
certain tissues. Very lipid-soluble substances,
such as thiopental, can build up in fat. Mepacrine,
an antimalarial drug, has a concentration in the
liver 200 times that in the plasma because it binds
to nucleic acids. Some drugs are even actively
transported into certain organs, for example, iodine
hormones accumulate in the thyroid.
Drug metabolism
Before being excreted from the body, most drugs are me-
tabolized. A small number of drugs exist in their fully ion-
ized form at physiological pH (7.4) and, owing to this highly
polar nature, are metabolized to only a minor extent, if at
all. The sequential metabolic reactions that occur have been
categorized as phases 1 and 2.
Anaesthetists need to consider the weight of
their patient before administering thiopental
given that it is a highly lipid soluble medication
that will accumulate in the fat of obese patients
and thus have a longer half-life than in a thinner
patient.
Gastric juice
pH 3
Aspirin
Weak acid
p K a
3.
Pethidine
Weak base
p K a
8.
Plasma
pH 7.
Urine
pH 8
Anion
A
Undissociated
acid
AH
100
100
30
Free
base B
Protonated
base
BH
<0.
Ionisation greatest at acid pH
6
Ionisation greatest at alkaline pH
Relative concentration
Fig. 1.9 Theoretic partition of a weak acid (aspirin) and a weak base (pethidine) between aqueous compartments (urine,
plasma, and gastric juice) according to the pH difference between them. (From Rang HP, Dale MM, Ritter JM, Moore PK.
Pharmacology. 8
th
ed. Edinburgh: Churchill Livingstone, 2016.)
Pharmacokinetics 11
The liver is the major site of drug metabolism although
most tissues can metabolize specific drugs. Other sites of
metabolism include the kidney, the lung and the gastroin-
testinal tract. Diseases of these organs may therefore affect a
drug's pharmacokinetics.
Orally administered drugs, which are usually absorbed
in the small intestine, reach the liver via the portal circula-
tion. At this stage, or within the small intestine, the drugs
may be extensively metabolized; this is known as the first-
pass metabolism and means that considerably less drug
reaches the systemic circulation than enters the portal vein
(see Fig. 1.10). This causes problems because it means that
higher doses of the drug must be given and, owing to indi-
vidual variation in the degree of the first-pass metabolism,
the effects of the drug can be unpredictable. Drugs that are
subject to a high degree of the first-pass metabolism, such
as the local anaesthetic lidocaine, cannot be given orally and
must be administered by some other route.
Phase 1 metabolic reactions include oxidation, reduction
and hydrolysis. These reactions introduce a functional
group, such as OH
or NH 2
, which increases the polar-
ity of the drug molecule and provides a site for phase 2
reactions.
Oxidations are the most common type of reaction and are
catalysed by an enzyme system known as the microsomal
mixed function oxidase system, which is located on the
smooth endoplasmic reticulum. The enzyme system forms
small vesicles known as microsomes when the tissue is
homogenized.
other enzymes are involved. This enzyme is a haemoprotein
that requires the presence of oxygen, reduced nicotinamide
adenine dinucleotide phosphate (NADPH) and NADPH
cytochrome P 450 reductase to function.
Spleen
Stomach
Hepatic portal
Liver
Hepatic
Inferior vena cava
Cystic
Gallbladder
Duodenum
Pancreaticoduodenal
Pancreas
Transverse colon
Superior mesenteric
Middle colic
Jejunal and ileal
Right colic
Ascending colon
Ileocolic
Ileum
Cecum
Appendix
Drain into superior mesenteric vein
Drain into splenic vein
Drain into inferior mesenteric vein
Short gastric
Left gastric
Pancreatic
Left gastroepiploic
Right gastric
Splenic
Left colic
Inferior mesenteric
Descending colon
Sigmoidal
Sigmoid colon
Superior rectal
Rectum
Right gastroepiploic
Fig 1.10 Portal venous system.
Pharmacokinetics 11
function oxidases. This results in the formation of the toxic
metabolite N-acetyl-p-benzoquinone which is inactivated
by glutathione. However, when glutathione is depleted, this
toxic metabolite reacts with nucleophilic constituents in the
cell leading to necrosis in the liver and kidneys.
N-Acetylcysteine or methionine can be administered in
cases of paracetamol overdose, because these increase liver glu-
tathione formation and the conjugation reactions, respectively.
Drug excretion
Drugs are excreted from the body in a variety of different
ways. Excretion predominantly occurs via the kidneys into
urine or by the gastrointestinal tract into bile and faeces.
Volatile drugs are predominantly exhaled by the lungs into
the air. To a lesser extent, drugs may leave the body through
breast milk and sweat.
The volume of plasma cleared of drug per unit time is
known as the clearance.
Glomerular filtration, tubular reabsorption (passive and
active), and tubular secretion all determine the extent to
which a drug will be excreted by the kidneys.
Glomerular capillaries allow the passage of molecules with
a molecular weight less than 20 000. The glomerular filtrate
thus contains most of the substances in plasma except proteins.
the corpuscular membrane repels negatively charged
molecules, including plasma proteins.
albumin will not be filtered.
Most of the drug in the blood does not pass into the glo-
merular filtrate but passes into the peritubular capillaries of
the proximal tubule where, depending on its nature, one of
two transport mechanisms will transport it into the lumen
of the tubule. One transport mechanism deals with acidic
molecules, the other with basic molecules.
responsible for most of the drug excretion carried
out by the kidneys and, unlike glomerular filtration,
allows the clearance of drugs bound to plasma proteins.
Competition between drugs that share the same
transport mechanism may occur, in which case the
excretion of these drugs will be reduced.
of molecules in the ionized state, which is in turn
dependent on the pH of the urine.
The extent to which excretion is impaired can be
deduced by measuring 24-hour creatinine clearance.
Some drug conjugates are excreted into the bile and subse-
quently released into the intestines where they are hydro-
lysed back to the parent compound and reabsorbed. This
“enterohepatic circulation” prolongs the effect of the drug.
Mathematic aspects of
pharmacokinetics
Two types of kinetics, related to the plasma concentra-
tion of a drug, describe the rate at which a drug leaves
the body.
in drug levels in the body that is independent of the
plasma concentration, and the rate is held constant by a
limiting factor, such as a cofactor of enzyme availability.
The liver is the main site of drug inactivation, and
the kidneys and gastrointestinal tract the main sites
for drug excretion. Disease of these organs will
alter the pharmacokinetics of a drug.
time
Plasma concentration
of drug
Zero order
t 1/
A
Time
Plasma concentration
of drug
First order
B
t 1/
Fig. 1.11 Plasma drug concentration versus time plot. (A) For a drug displaying zero-order kinetics. (B) For a drug
displaying first-order kinetics. t ½ , half-life.
When the plasma concentration is plotted against time,
the decrease is a straight line. Alcohol is an example of
a drug that displays zero-order kinetics.
drugs. It describes a decrease in drug levels in the
body that is dependent on the plasma concentration
because the concentration of the substrate (drug) is the
rate-limiting factor. When the plasma concentration is
plotted against time, the decrease is exponential.
The one-compartment model usually gives an adequate
clinical approximation of drug concentration by consider-
ing the body to be a single compartment. Within this single
compartment, a drug is absorbed, immediately distributed,
and subsequently eliminated by metabolism and excretion.
If the volume of the compartment is V d and the dose
administered D, then the initial drug concentration, C o
will be:
o d
The time taken for the plasma drug concentration to fall
to half of its original value is the half-life of that drug. The
decline in concentration may be exponential, but this situ-
ation expresses itself graphically as a straight line when the
log plasma concentration is plotted against the time after
intravenous dose (Fig. 1.12A).
Half-life is related to the elimination rate constant (K el
by the following equation:
t K natural ln 1 2 el
/
× = log ( )
Half-life is related to V d , but does not determine the ability
of the body to remove the drug from the circulation, be-
cause both V d and half-life change in the same direction.
The body's ability to remove a drug from the blood is
termed clearance (Cl p ) and is constant for individual drugs.
Cl V K p d el
If the drug is not administered parenterally, plotting the
log plasma drug concentration against time will require the
consideration of both absorption and elimination from the
compartment (Fig. 1.12B).
The one-compartment model is widely used to deter-
mine the dose of the drug to be administered. The two-
compartment model expands on this model by considering
the body as two compartments to allow some consideration
of drug distribution.
For drugs displaying first-order kinetics, the level of the
drug in the body increases until it is equal to the level ex-
creted, at which point steady-state is reached (Fig. 1.13),
such that:
to five half-lives.
depend upon the frequency of drug administration:
the greater the frequency, the greater the amount of
drug and the less the variation between peak and
trough plasma concentrations. If the frequency of
administration is greater than the half-life, then an
accumulation of the drug will occur.
The loading dose can be calculated according to the de-
sired plasma concentration at steady-state (C ss
) and the vol-
ume of distribution (V d
) of the drug:
Loading dose mg kg V L kg C mg L d ss
Adherence
Lastly, despite not being a pharmacological property, it is im-
portant to consider adherence. For some drugs to be effec-
tive (e.g. antibiotics), they must be taken at regular intervals
and for a certain period of time. Adherence can be an issue
in paediatric and elderly patients. With children, parents
must remember to give the medicine and follow directions
Time
5 10
10
1
15 20 25 50
t 1/
Log plasma concentration
of drug
Slope = K el
C o
A
0 5 10 15 20 25
Time
C o
Slope = K el
Log concentration
B
Fig. 1.12 Log plasma drug concentration versus time plot for a drug compatible with the one-compartment open
pharmacokinetic model for drug disposition. (A) After a parenteral dose, assuming first-order kinetics. (B) After an oral
dose. C o,
initial drug concentration; K el
, elimination rate constant. (modified from Page, C., Curtis, M. Walker, M, Hoffman,
B. (eds) Integrated Pharmacology, 3rd edn. Mosby, 2006.)
Adverse effects
As well as interacting with one another and with their target
tissue, drugs will also interact with other tissues and organs
and alter their function. No drug is without side effects, al-
though the severity and frequency of these will vary from
drug to drug and from person to person.
The liver and the kidneys are susceptible to the ad-
verse effects of drugs, as these are the sites of drug metab-
olism and excretion. Some drugs cause hepatotoxicity or
nephrotoxicity.
Some people are more prone to the adverse effects of
drugs.
medications that are teratogenic, that is, cause foetal
malformations (e.g. thalidomide taken in the 1960s for
morning sickness).
drugs they take, because many drugs can be passed on
in the breast milk to the developing infant.
or kidney disease. These illnesses will result in
decreased metabolism and excretion of the drug and
will produce the side effects of an increased dose of the
same drug.
drugs have an increased risk of drug interactions and
the associated side effects. In addition, elderly patients
have a reduced renal clearance and a nervous system
that is more sensitive to drugs. The dose of drug
initially given is usually 50% of the adult dose, and
certain drugs are contraindicated.
toxicity because of immature clearance systems.
6-phosphate dehydrogenase deficiency. The deficiency
will result in haemolysis if an oxidant drug, such as
aspirin, is taken.
Certain drugs are carcinogenic, that is, induce cancer.
Allergic reactions to certain drugs are common, occurring in
2% to 25% of cases. Most of these are not serious, for exam-
ple, skin reactions; however, rarely, reactions such as anaphy-
lactic shock (type 1 hypersensitivity) occur that may be lethal,
unless treated with intramuscular adrenaline. The most com-
mon allergic reaction is to penicillin, which produces an ana-
phylactic shock in approximately 1 in 50,000 people.
Drug history
A patient's drug history is a crucial component of the clerk-
ing process, because drug effects account for a significant
proportion of hospital admissions, and potential drug inter-
actions and adverse events are crucial to foresee.
A complete list of the names and doses of prescribed
drugs taken by the patient (noting the proprietary and
the generic name, for example, Viagra and sildenafil, re-
spectively) and any other medications or supplements
they may have bought themselves over the counter at a
pharmacy should be documented. Women often forget
the contraceptive pill and hormone replacement ther-
apy and should be sensitively questioned about these.
NSAIDs and paracetamol are often taken by patients
with arthritis and should be specifically asked about.
Make sure to note how often the drugs were taken, and
at what times.
If presented with numerous bottles and packets of tab-
lets, ensure they all belong to the patient, and not the part-
ner of the patient, or to someone else. Always ask the patient
if they are taking all their medicines as prescribed.
Occasionally, it is useful to know what drugs have been
taken in the recent and distant past; for example, mono-
amine oxidase inhibitors should be stopped at least 3 weeks
before starting a different antidepressant therapy.
Previous adverse reaction to drugs, and to nondrug
products such as latex, is essential to ascertain. Explore what
happened to the patient, and what was done about it. An
upset stomach a day after taking penicillin is a common side
effect, and is not grounds for choosing another antibiotics
when treating a penicillin-sensitive infection in the future.
A widespread cutaneous rash and difficulty breathing
which required adrenaline and a hospital admission sug-
gests an allergic drug reaction and therefore this, or any re-
lated drug should be clearly avoided in the future. Allergy
to drugs should be clearly marked in the patient's notes and
drug charts.
The family history of adverse drug reactions is usually
confined to the anaesthetic history, where the concern is
largely in relation to the muscle-relaxing drugs, particularly
suxamethonium.
A history of recreational or illicit drug use is an import-
ant but sensitive issue to approach. One must use discretion
when questioning the patient. A history of smoking should
also be established.
Knowledge about any hepatic or renal disease and gen-
eral health problems is important when it comes to man-
agement and prescribing, as are specific considerations,
such as not prescribing aspirin in peptic ulcer disease, or
oestrogen to patients with oestrogen-dependent cancers.
These aspects are usually brought to light in the rest of the
history taking.
Adverse reactions and allergy to a drug are
different. Adverse reactions are usually minor
irritations, whereas an allergic reaction can be life
threatening.
Drug history and drug development 11
Drug development
Hundreds of thousands of substances have been produced
by the pharmaceutical industry over the past 50 years, al-
though very few ever get past preclinical screening, and
fewer than 10% of these survive clinical assessment.
There are four stages a potential drug goes through from
discovery to being approved (Table 1.4).
Phase 4 can be regarded as an ongoing phase, where
drugs are monitored once licensed for general use. By
this stage, the efficacy and dose–response relationship are
known, although the side-effect profile is often incomplete,
and information is gathered on these “adverse reactions”
which are caused by, or likely caused by new drugs.
In the United Kingdom, this is known as the yellow card
scheme. The British National Formulary (BNF) contains
detachable yellow cards, which medical staff complete, doc-
umenting adverse drug reactions in their patients, which
can then be forwarded to the Medicines Control Agency.
The Medicines Control Agency collates these data and uses
them for surveillance of common or severe adverse effects.
The data are publicized in future copies of the BNF, or used
in the reassessment of certain drug licences.
The salient points of the drug history are:
Phase
Main aims/means
of investigation Subjects
Preclinical Pharmacology In vitro
Toxicology In laboratory
animals
Phase 1 Clinical
pharmacology and
toxicology
Healthy individuals
and/or patients
Drug metabolism
and bioavailability
Evaluate safety
Phase 2 Initial treatment
studies
Small numbers of
patients
Evaluate efficacy
Phase 3 Large randomized
controlled trials
Large numbers of
patients
Comparing new to
old treatments
Evaluate safety and
efficacy
Phase 4 Postmarketing
surveillance
All patients
prescribed the
drug
Long-term safety
and rare events
Yellow card
scheme
Table 1.4 The five stages of drug development and
monitoring
The majority act via receptors in cell membranes but they can also work on transporter
molecules and enzymes.
depolarization. Interaction with G protein-coupled receptors (metabotropic) results in
secondary messenger involvement and either calcium release or protein phosphorylation.
Kinase-linked receptor activation results in protein phosphorylation which induces gene
transcription and protein synthesis. Nuclear receptor activation results in gene transcription
and protein synthesis.
dosage can be modelled by pharmacokinetics to relate to plasma concentration of a drug.
Adverse drug effects stem from the drug interacting with tissues and organs to alter their
function. Adverse reactions are usually minor, whereas allergic reactions can be life-threatening.
trials. Phase 4 is postmarketing surveillance and is always ongoing once the drug is in the market.