












































































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
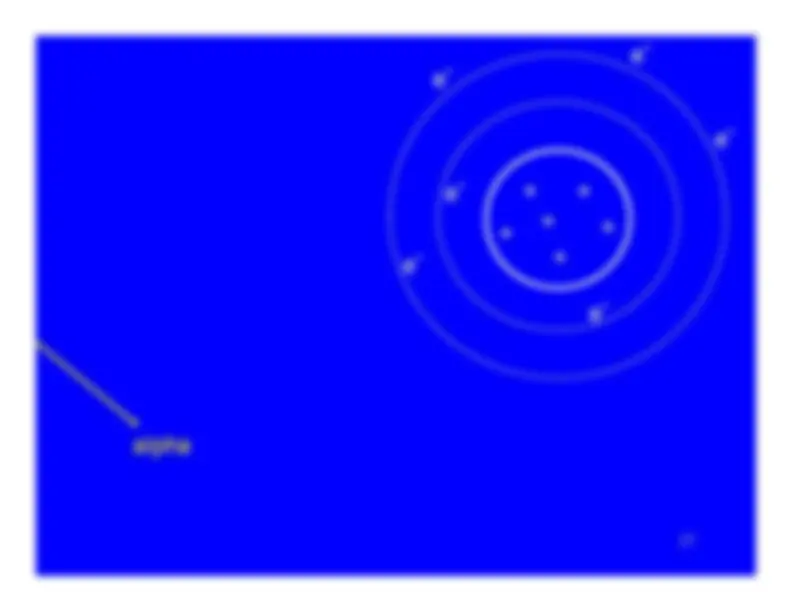
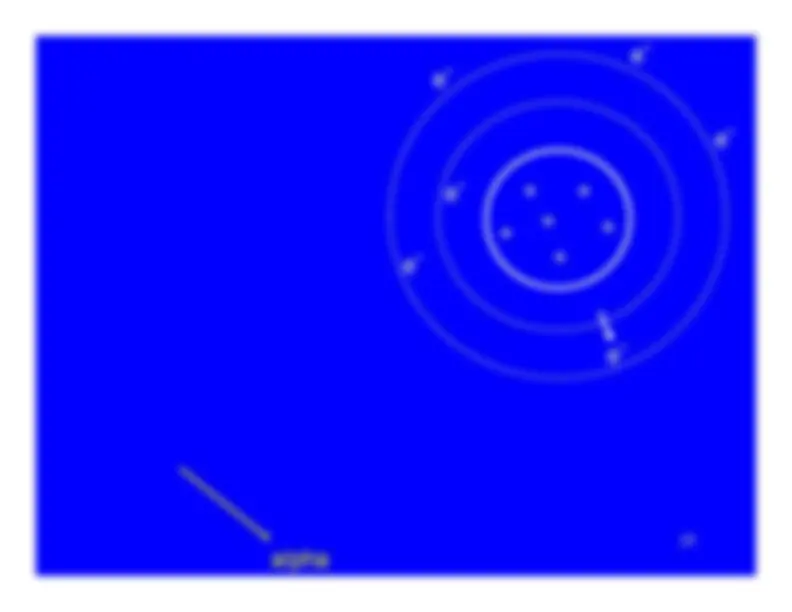
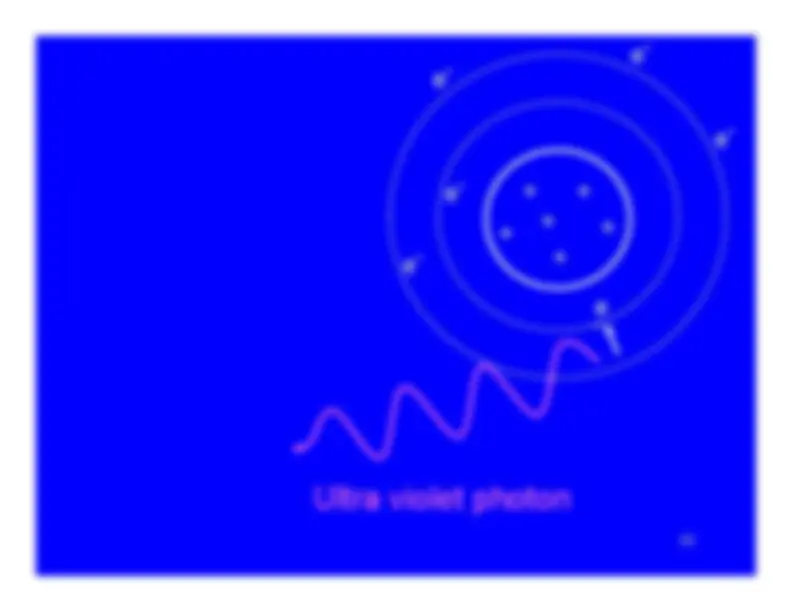
The charged particle (alpha or beta particle) exerts just enough force to promote one of the atom's electrons to a higher energy state (shell).
Typology: Summaries
1 / 96

This page cannot be seen from the preview
Don't miss anything!

























































































3/1/
Objectives
QualitativeQuantitative Introduction
GeneralForce of the Interaction
Force of the InteractionFour Types of Charged Particle Interactions Ionization
GeneralIon PairsDelta Ray
Quantitative Measures of Energy Loss
GeneralW ValueSpecific IonizationStopping Power and Linear Energy TransferMass Stopping Power
Alpha Particles
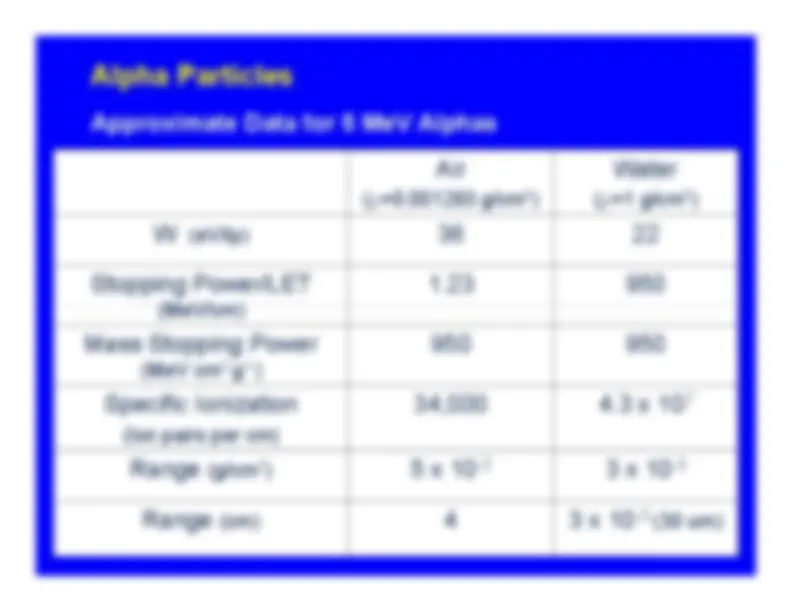
GeneralAlpha TracksRangeRange in AirApproximate Data for 5 MeV Alphas
Beta Particles
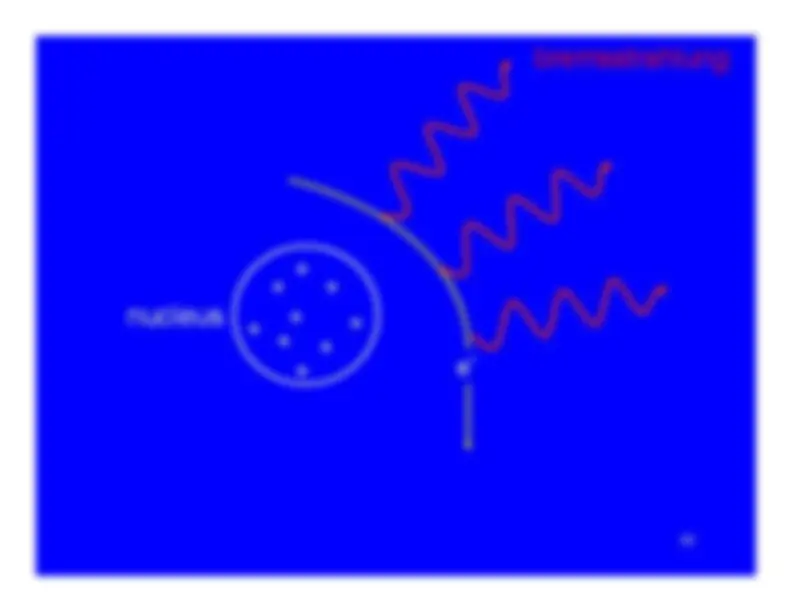
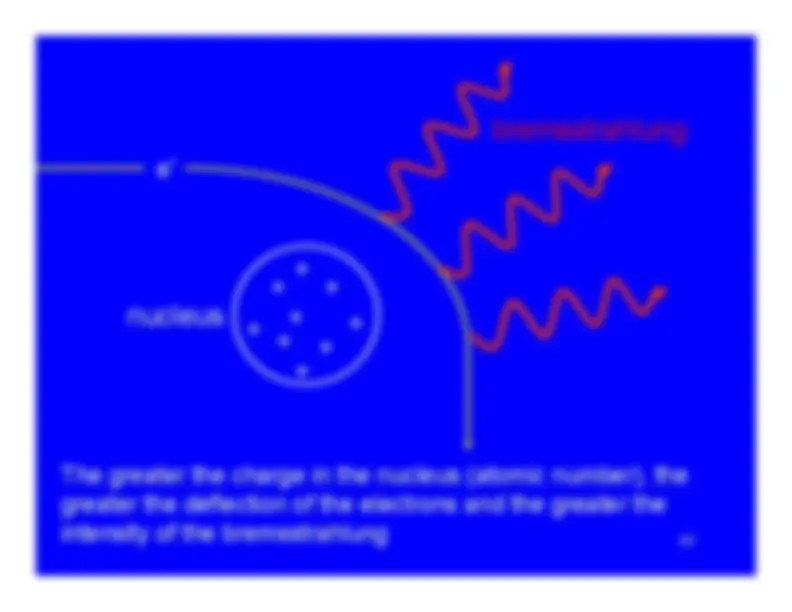
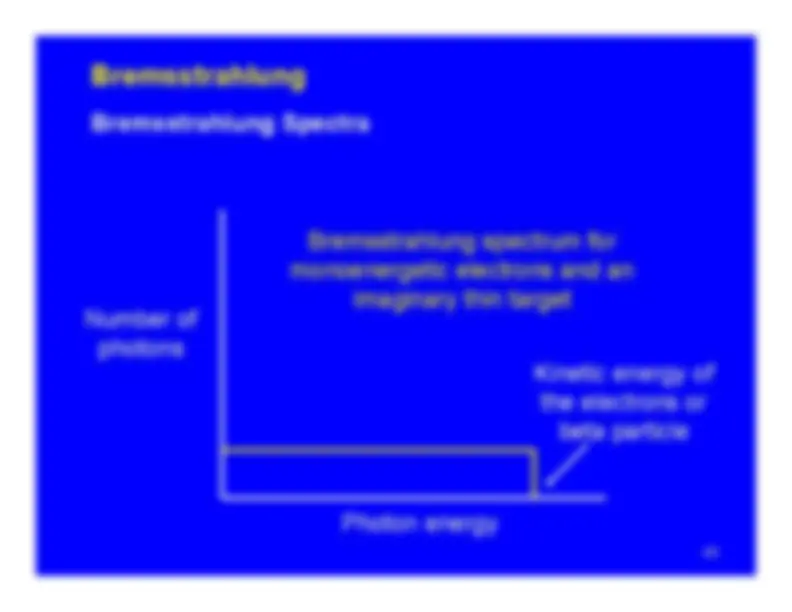
GeneralRangeRange (as a density thickness)Range and PenetrationBremsstrahlungC
k^
R di ti
Cerenkov RadiationApproximate Data for 1 MeV Beta Particles Summary
Types of InteractionsAlpha ParticlesBeta Particles References
To review the following measures of energy loss:
“The interaction of charged particles with matter”concerns the transfer of energy from the chargedparticles to the material through which they travel.
-^
The “charged particles” considered here are:
The
charged particles
considered here are:
Photons and neutrons, which have no charge,interact very differently.
Charged particles passing through matter continuouslyinteract with the electrons and nuclei of the surroundingatoms.
-^
In other words, alpha and beta particles are continually
slowing down as they travel through matter.
-^
The interactions involve the electromagnetic forces ofattraction or repulsion between the alpha or betaparticles and the surrounding electrons and nuclei.
Things to notice about the equation:
quadruples if the distance is cut in half)
q
p^
repulsive)
Ionization (alphas and betas)Excitation (alphas and betas)Bremsstrahlung (primarily betas) Bremsstrahlung (primarily betas)Cerenkov radiation (primarily betas)
energy loss.
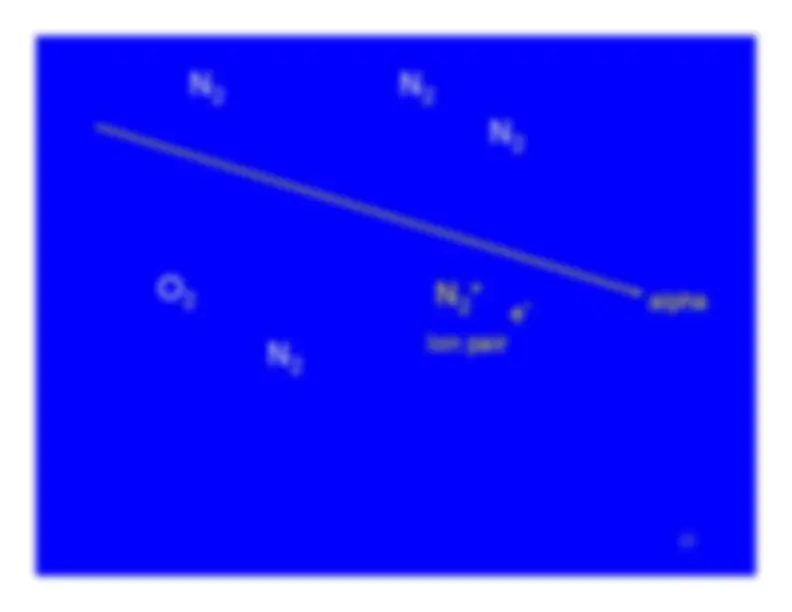
A charged particle (alpha or beta particle) exertssufficient force of attraction or repulsion to completelyremove one or more electrons from an atom.
-^
The energy imparted to the electron must exceed thebi di
f th
l^
t
binding energy of the electron.
-^
Ionization is most likely to involve atoms near thecharged particle's trajectory.
-^
Each ionization event reduces the charged particle'svelocity, i.e., the alpha or beta particles loses kineticenergy.
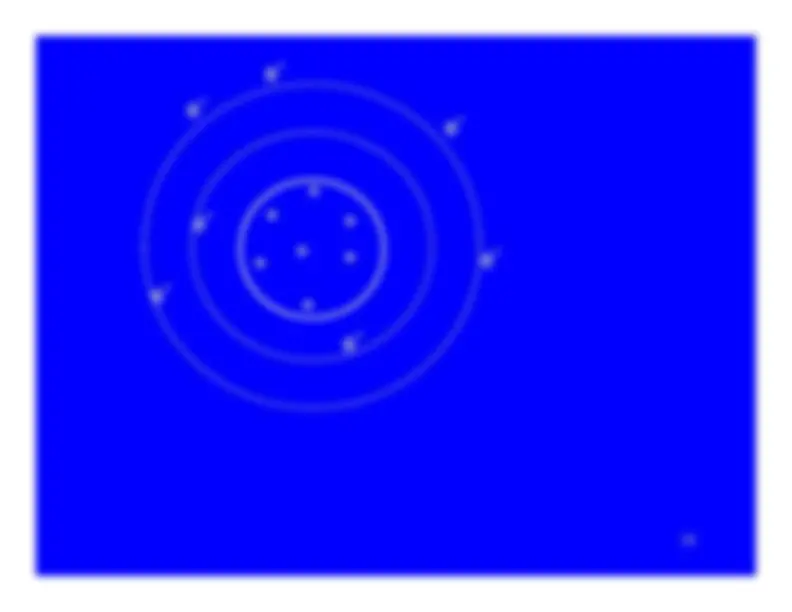
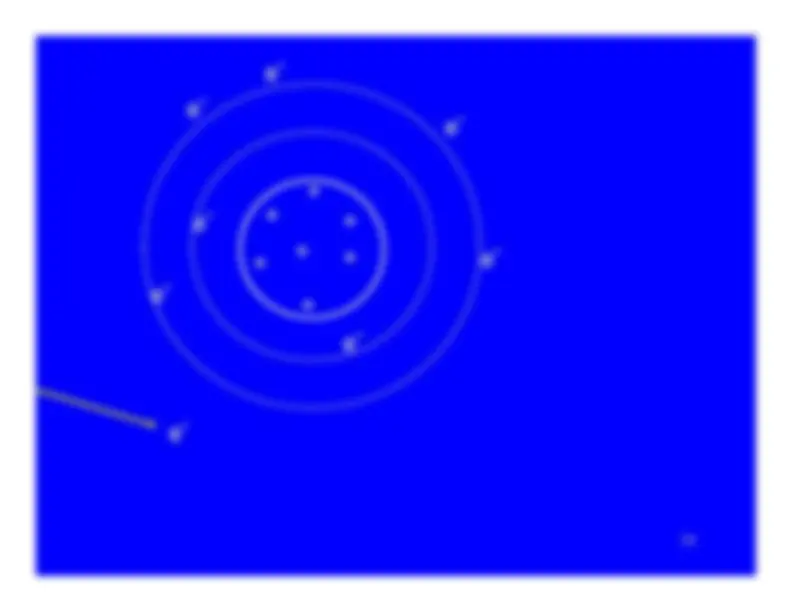
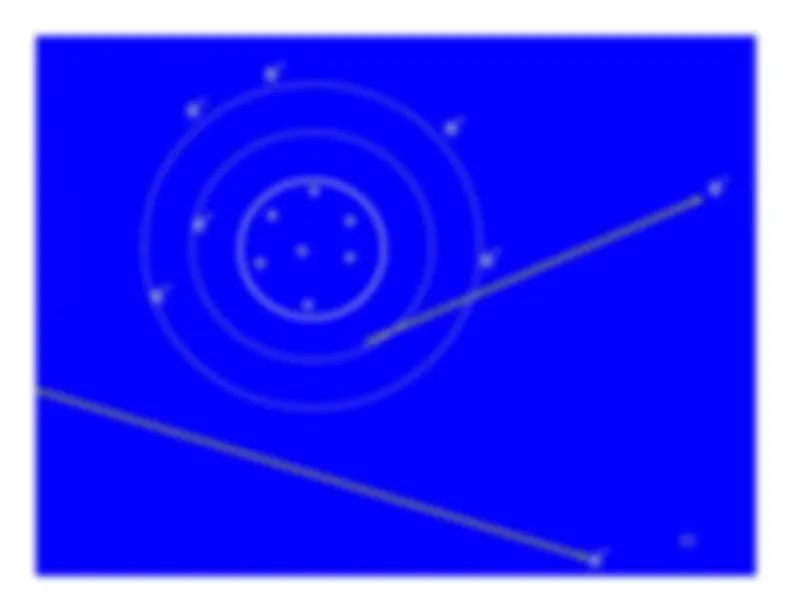
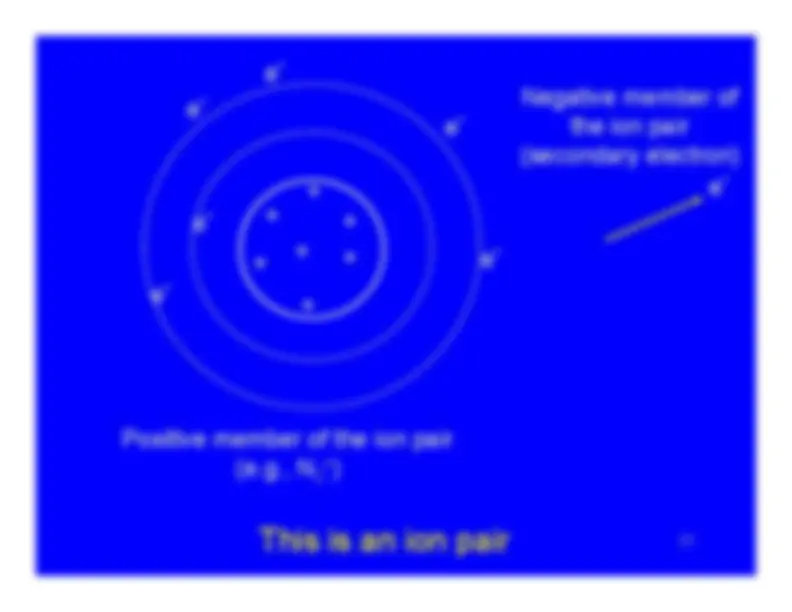
Ionization turns a neutral atom into an ion pair.
-^
The electron stripped away from the atom is the negativemember of the ion pair.It is known as a secondary electron.
y
The secondary electron has some, but not much, kineticenergy - usually less than 100 eV.Sometimes it has enough energy to ionize additionalatoms. Then it is referred to as a delta ray.
-^
The atom , now with a vacancy in one of its electronshells, is the positive member of the ion pair.
20