





























Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
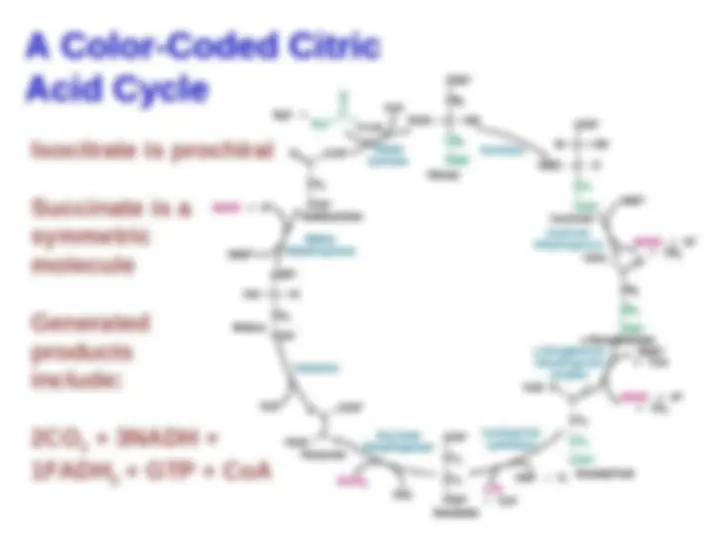
An in-depth look into the citric acid cycle, focusing on the roles of citrate synthase and succinyl coa synthetase. It explains the mechanism of citrate synthesis, the importance of ordered binding, and the significance of high phosphoryl-transfer potential compounds in the cycle. Additionally, it discusses the structure and function of succinyl coa synthetase and the regeneration of oxaloacetate.
Typology: Study notes
1 / 46

This page cannot be seen from the preview
Don't miss anything!







































© 2010 W. H. Freeman and Company
Citric Acid Cycle Generates Reductive
Equivalents Utilized in Respiration
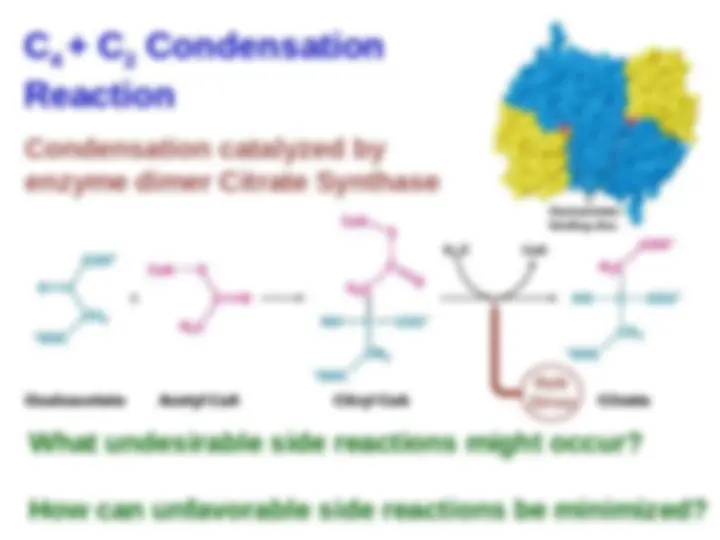
Mechanism of Synthesis of Citryl CoA
Mechanism of Synthesis of Citryl CoA
by
by Citrate Synthase.
Citrate Synthase.
The condensation
The condensation
of oxaloacetate and acetyl CoA
of oxaloacetate and acetyl CoA
proceeds through an
proceeds through an enol
enol
intermediate
intermediate
. The subsequent . The subsequent
hydrolysis of citryl CoA yields
hydrolysis of citryl CoA yields
citrate and CoA.
citrate and CoA.
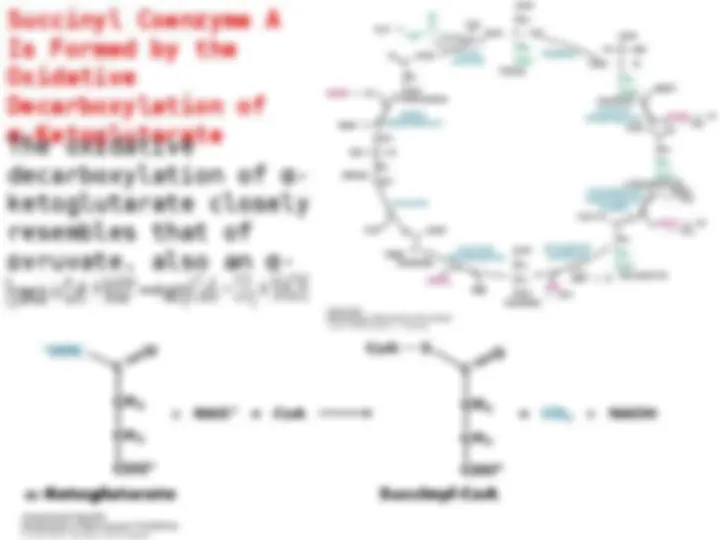
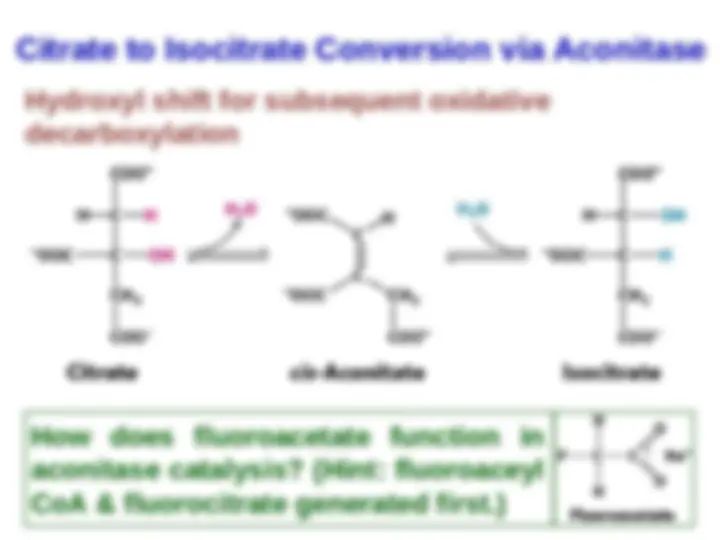
ocitrate Is Oxidized and
ocitrate Is Oxidized and
Decarboxylated Decarboxylated toto α-α-
Ketoglutarate Ketoglutarate
now now toto thethe firstfirst ofof fourfour
oxidation-reduction oxidation-reduction reactionsreactions
in in thethe citriccitric acidacid cycle.cycle. TheThe
oxidative oxidative decarboxylationdecarboxylation ofof
isocitrate isocitrate isis catalyzedcatalyzed byby
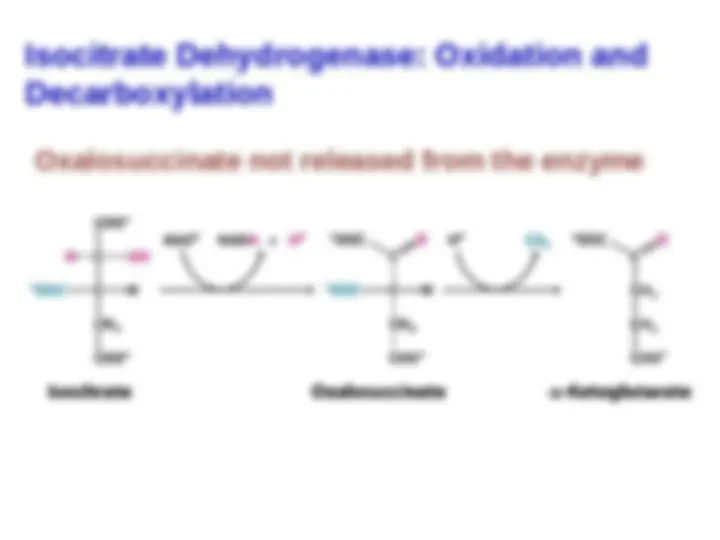
isocitrate isocitrate dehydrogenasedehydrogenase.. TheThe
intermediate intermediate inin thisthis reactionreaction
is
is oxalosuccinate,
oxalosuccinate, an
an
unstable β-ketoacid. While
unstable β-ketoacid. While
bound to the enzyme, it
bound to the enzyme, it loses
loses
22
to form
to form α-ketoglutarate.
α-ketoglutarate.
This oxidation generates the
This oxidation generates the
first high-transfer-potential
first high-transfer-potential
electron carrier NADH in the
electron carrier NADH in the
cycle.
cycle.
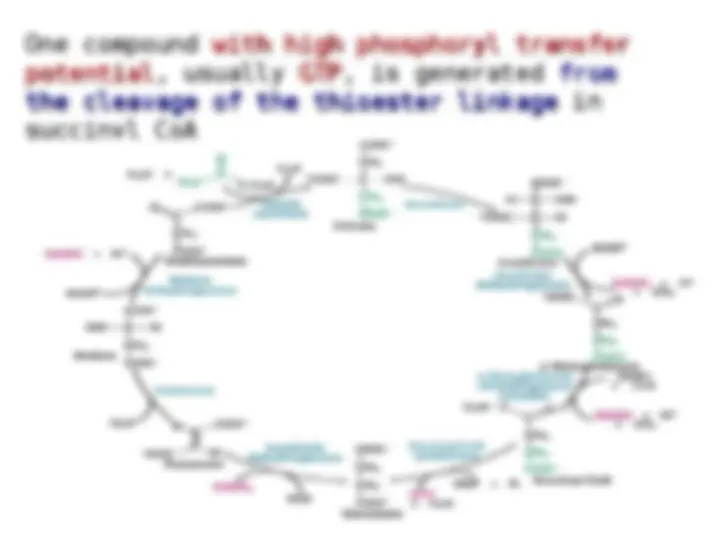
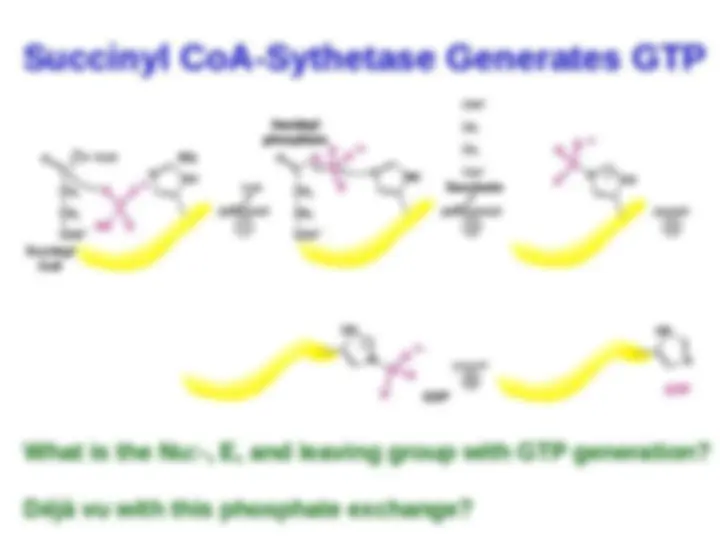
cleavage of the thioester bond
cleavage of the thioester bond
powers the synthesis of the
powers the synthesis of the
six-carbon citrate
six-carbon citrate from the
from the
four-carbon oxaloacetate
four-carbon oxaloacetate and
and
the
the two-carbon fragment.
two-carbon fragment. The
The
cleavage of the thioester bond
cleavage of the thioester bond
of succinyl CoA is coupled to
of succinyl CoA is coupled to
the phosphorylation of a
the phosphorylation of a
purine nucleoside diphosphate,
purine nucleoside diphosphate,
usually GDP.
usually GDP.
This reaction is catalyzed by
This reaction is catalyzed by
succinyl CoA synthetase
succinyl CoA synthetase
(succinate thiokinase). Some
(succinate thiokinase). Some
mammalian
mammalian succinyl CoA
succinyl CoA
synthetases
synthetases are specific
are specific
for
for GDP and others for ADP
GDP and others for ADP .
The
The E. coli
E. coli enzyme uses either
enzyme uses either
GDP or GDP as the
GDP or GDP as the phosphoryl-
phosphoryl-
group acceptor
group acceptor .
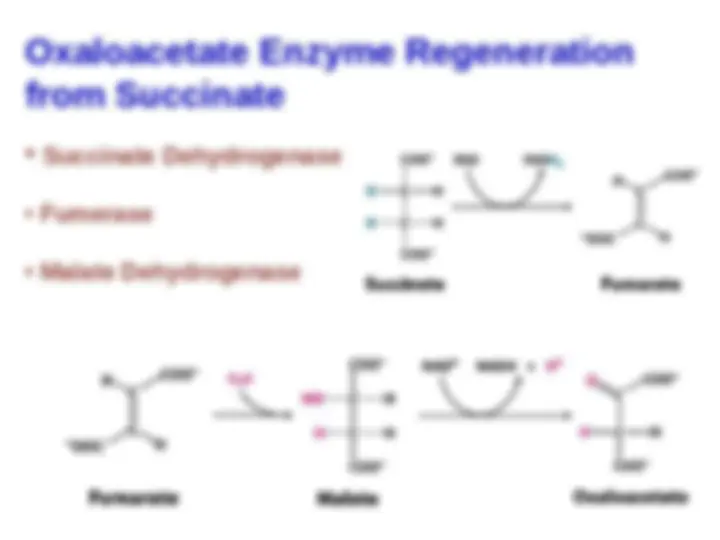
Oxaloacetate Is Regenerated by the Oxidation of
Oxaloacetate Is Regenerated by the Oxidation of
Succinate
Succinate
Reactions of four-carbon compounds constitute the final
Reactions of four-carbon compounds constitute the final
stage of the citric acid cycle: the regeneration of
stage of the citric acid cycle: the regeneration of
oxaloacetate. A methylene group (CH
oxaloacetate. A methylene group (CH
22
) is converted into a
) is converted into a
carbonyl group (C = O) in three steps: an oxidation, a
carbonyl group (C = O) in three steps: an oxidation, a
hydration, and a second oxidation reaction. Not only is
hydration, and a second oxidation reaction. Not only is
oxaloacetate thereby regenerated for another round of
oxaloacetate thereby regenerated for another round of
the cycle, but also more energy is extracted in the form
the cycle, but also more energy is extracted in the form
of FADH
of FADH
2
2
and NADH.
and NADH.
Succinate is oxidized to fumarate by
Succinate is oxidized to fumarate by succinate
succinate
dehydrogenase
dehydrogenase .
The hydrogen acceptor is FAD. In
The hydrogen acceptor is FAD. In
succinate dehydrogenase, the isoalloxazine ring of FAD
succinate dehydrogenase, the isoalloxazine ring of FAD
is covalently attached to a histidine side chain of the
is covalently attached to a histidine side chain of the
enzyme (denoted E-FAD).
enzyme (denoted E-FAD).
FAD is the hydrogen acceptor
FAD is the hydrogen acceptor
in this reaction because the
in this reaction because the
free-energy change is
free-energy change is
insufficient to reduce NAD
insufficient to reduce NAD
++
FAD is nearly always the
FAD is nearly always the
electron acceptor in
electron acceptor in
oxidations that remove two
oxidations that remove two
hydrogen
hydrogen atoms
atoms from a
from a
substrate.
substrate.
Succinate dehydrogenase
Succinate dehydrogenase , like
, like
aconitase
aconitase , is an
, is an iron-sulfur
iron-sulfur
protein
protein
. Indeed, succinate . Indeed, succinate
dehydrogenase contains three
dehydrogenase contains three
different kinds of
different kinds of iron-sulfur
iron-sulfur
clusters, 2Fe-2S
clusters, 2Fe-2S (two iron
(two iron
atoms bonded to two inorganic
atoms bonded to two inorganic
sulfides), 3Fe-4S, and 4Fe-4S.
sulfides), 3Fe-4S, and 4Fe-4S.
Succinate dehydrogenase—which
Succinate dehydrogenase—which
consists of two subunits, one
consists of two subunits, one
70 kd and the other 27
70 kd and the other 27 kd—
kd—
differs from other enzymes in
differs from other enzymes in
the citric acid cycle in being
the citric acid cycle in being