










Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Lab questions for final exam on general chemistry. 2015 SU CHE 111 Final Exam - Confidential
Typology: Lab Reports
1 / 18

This page cannot be seen from the preview
Don't miss anything!











Your Name: Your ID:
# of Questions: 12
Date and Time of Exam Creation: Fri, Jul 24, 2015 @ 11:10:
Total Exam Points: 160.
attachment_for_pubExamUID_lnxp114377506216281436XX_11.jpg
Question #: 1
Which of the following equipment setups would you use for a calorimetry experiment?
A.
B.
C.
D.
Question #: 3
Which of the following provides an appropriate level of eye protection from fumes and splashes for use in the General Chemistry Lab?
A.
B.
C.
D.
Question #: 4
Question #: 5
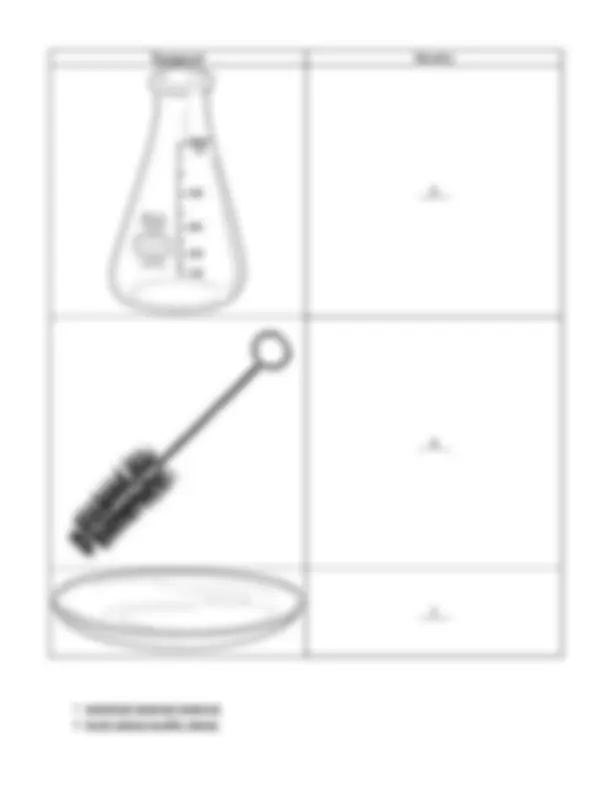
Identify the following pieces of laboratory equipment.
Equipment Identity
Question #: 6
A. 2.86 g/mL B. 10.8 g/mL C. 11.8 g/mL D. 3.74 g/mL
Question #: 7
What is the net ionic equation for the reaction between the reagents shown below? magnesium chloride + sodium hydroxide Complete the table shown below with your response.
SubstanceState SubstanceState SubstanceState 1 ( 2 ) + 3 ( 4 ) → 5 ( 6 ) You do not need to balance the reaction.
Question #: 8 Consider the reactions N 2 H 4 ( l ) + CH 4 O( l ) → CH 2 O( g ) + N 2 ( g ) + 3 H 2 ( g ) ∆H = – 37 kJ N 2 ( g ) + 3 H 2 ( g ) → 2 NH 3 ( g ) ∆H = – 46 kJ CH 4 O( l ) →CH 2 O( g ) + H 2 ( g ) ∆H = – 65 kJ The ∆H for the overall reaction shown below is 1 kJ/mol. Do not include units in your answer. N 2 H 4 ( l ) + H 2 ( g ) → 2 NH 3 ( g )
Question #: 9
What is the percent composition of each element in (NH 4 ) 2 S? N: 1 % H: 2 % S: 3 %
Question #: 10
Write a brief procedure on how you would determine the concentration of citric acid (H 3 C 6 H 5 O 3 ) using sodium hydroxide and materials available in the lab. Your reponse will not be saved until you click the "save" icon.
Question #: 11
Salicylic acid (FW = 138.12 g/mol), a reagent used to make aspirin, can itself be produced from oil of wintergreen (methyl salicylate, FW = 152.15 g/mol, d = 1.18 g/mol at 20 ºC), by reacting it with sodium hydroxide. This reaction is shown below. You measured out 7.14 mL of oil of wintergreen and 9.59 g of sodium hydroxide (FW = 39.997 g/mol). Experimentally, you produced 6.48 g of salicylic acid. What is your percent yield for this reaction? C 8 H 8 O 3 + NaOH → C 7 H 6 O 3 + NaOCH 3
Your Name: Your ID:
# of Questions: 12
Date and Time of Exam Creation: Fri, Jul 24, 2015 @ 11:10:
Total Exam Points: 160.
attachment_for_pubExamUID_lnxp114377506216281436XX_11.jpg
Question #: 1
Which of the following equipment setups would you use for a calorimetry experiment?
A.
B.
C.
✓D.
Item Weight: 5.
Question #: 3
Which of the following provides an appropriate level of eye protection from fumes and splashes for use in the General Chemistry Lab?
A.
B.
C.
✓D.
Item Weight: 5.
Question #: 4
Item Weight: 20.
Question #: 5
Identify the following pieces of laboratory equipment.
Equipment Identity
Item Weight: 25.
Question #: 6
A. 2.86 g/mL B. 10.8 g/mL ✓C. 11.8 g/mL D. 3.74 g/mL
Item Weight: 5.
Question #: 7
What is the net ionic equation for the reaction between the reagents shown below? magnesium chloride + sodium hydroxide Complete the table shown below with your response.
SubstanceState SubstanceState SubstanceState 1 ( 2 ) + 3 ( 4 ) → 5 ( 6 ) You do not need to balance the reaction.
Item Weight: 30.
Question #: 8
Consider the reactions N 2 H 4 ( l ) + CH 4 O( l ) → CH 2 O( g ) + N 2 ( g ) + 3 H 2 ( g ) ∆H = – 37 kJ N 2 ( g ) + 3 H 2 ( g ) → 2 NH 3 ( g ) ∆H = – 46 kJ CH 4 O( l ) →CH 2 O( g ) + H 2 ( g ) ∆H = – 65 kJ The ∆H for the overall reaction shown below is 1 kJ/mol. Do not include units in your answer. N 2 H 4 ( l ) + H 2 ( g ) → 2 NH 3 ( g )
Item Weight: 5.
Question #: 9
What is the percent composition of each element in (NH 4 ) 2 S? N: 1 % H: 2 % S: 3 %
Item Weight: 15.
Question #: 10
Write a brief procedure on how you would determine the concentration of citric acid (H 3 C 6 H 5 O 3 ) using sodium hydroxide and materials available in the lab. Your reponse will not be saved until you click the "save" icon.
Item Weight: 30.