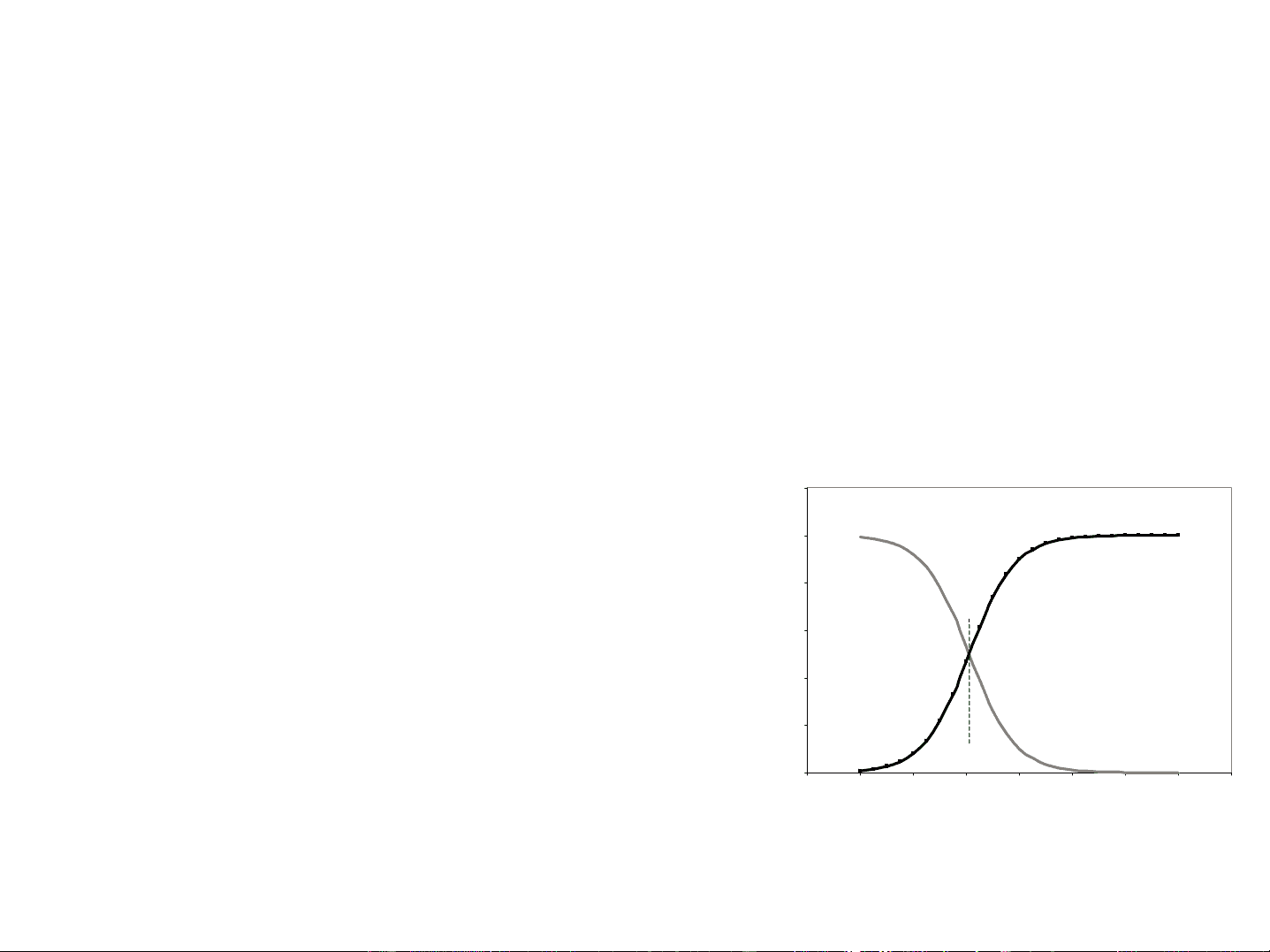






Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Why determine fractional compositions? Monoprotic Systems, Diprotic Systems and Calculating Fractional Compositions
Typology: Slides
1 / 8

This page cannot be seen from the preview
Don't miss anything!
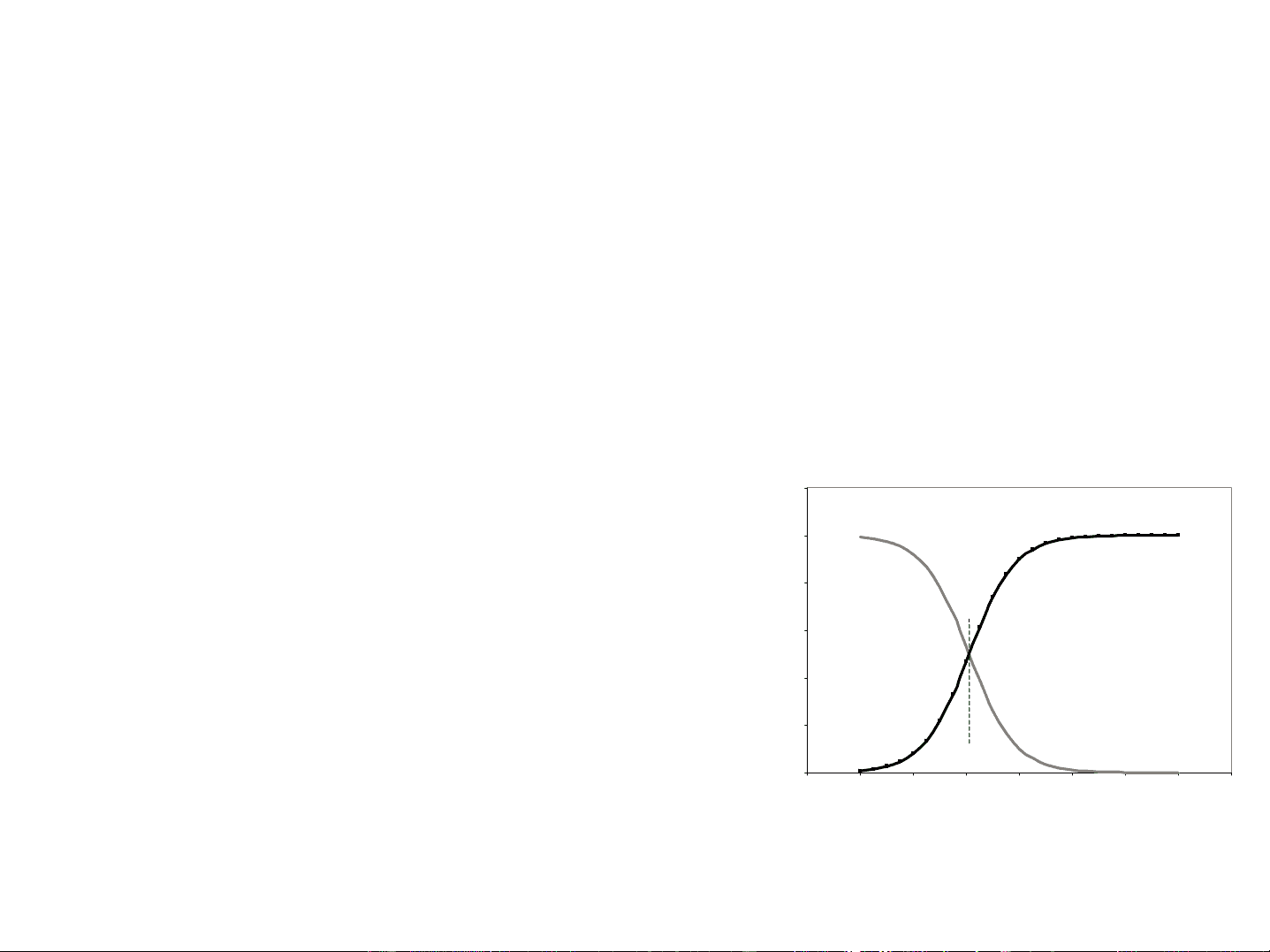




CHM 320 Lecture 18 Chap 11
Fractional composition clearly show the dominant and minorspecies in solution.
-^
Fractional compositions provide a way to know what reactionsare occurring, and what reactions are relatively unimportant.
-^
Fractional compositions make really cool plots. Monoprotic Systems For an acid -
α
HA
α
A-
For a base -
α
b^
α
BH+
F = Formal concentration
1.2^1 0.8 0.6 0.4 0.2^00
1
2
3
4
5
6
7
8
pH
fractional composition
alpha (HA)
alpha (A-)
pKa
CHM 320 Lecture 18 Chap 11
Diprotic Systems For an acid -
α
H2A
α
HA-
α
A2-
F = Formal concentration
1.2^1 0.8 0.6 0.4 0.2^00
1
2
3
4
5
6
7
8
pH
fractional composition
alph H2A
alph HA-
alph A-
pKa
pKa
CHM 320 Lecture 18 Chap 11
CHM 320 Lecture 18 Chap 11
Diprotic Buffers – treated similar to a monoprotic buffer. Youmust know what the desired pH and the appropriate pKa are. pH = pKa + log [base]/[acid]
CHM 320 Lecture 18 Chap 11
Calculate the pH of a 0.10 M solution of each amino acid. NH
2 C O CH
2 CH
2
O
glutamine
2
O
cysteine
2
2 CH
2 CH
2 CH
2
O
arginine
CHM 320 Lecture 18 Chap 11
Draw the structure and the fractional composition of the principalspecies of 1,3-dihydroxybenzene at the following pH values:
(pK
a
pK
a
pH 9 pH 11