







Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
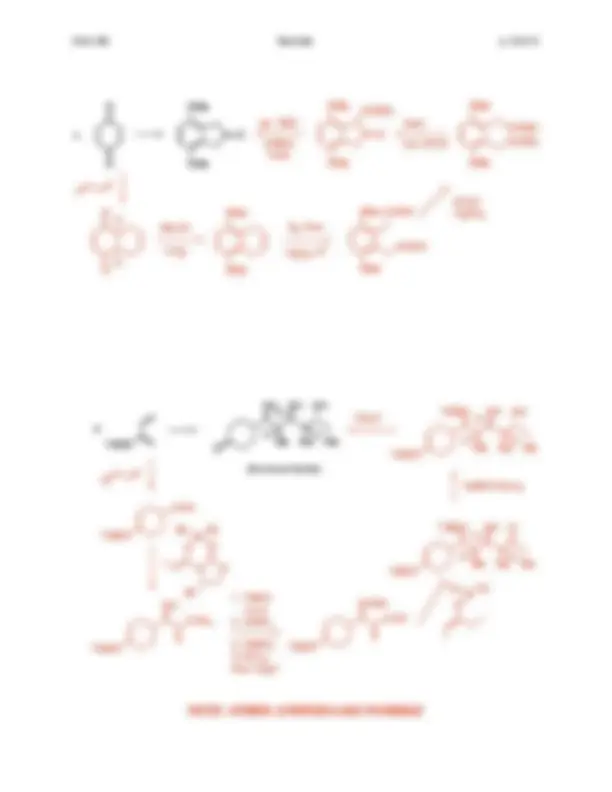
Complete this diagram by indicating all missing reagents / products. Each box corresponds to one reagent / product. Note: aqueous workup steps ...
Typology: Exams
1 / 12

This page cannot be seen from the preview
Don't miss anything!







2
1 OH
PhO
Cl
Et 2
2
1 O
OPh
Bu 3
SnH
2
1
Cl
Et 2
EtOH
reflux
OEt
(or any non-enolizable aldehyde)
NaOH
heat
2
1 OH
2
1
then Et 3
primary or
secondary
aldehyde
or ketone
seychellene
Se
Ph O
2
, Pd
Ph 3
2
The reaction required such a high temperature probably because :
Se
Ph O
together with
Krapcho
reaction
MeO OMe
Me
Me
Me
Me
Me
Me
Me
steps
Me
Me
Me
Me
Me
Me
Me
Me
Me
steps
Me
Me
Me
coriolin
COOMe
MeO OMe
MeO
OMe COOMe
aq. acid
(ketal hydrolysis)
NaOMe
(aldol-dehydration)
NaOMe
(aldol-dehydration)
Me
Me
Me
Me
a.
(or enantiomer)
Bn
2
3
MeOH
b.
Br
OMe
( R-config.)
2
=CHCu(PBu 3
2
c.
BCl
Et 3
d.
Ph
Ph
Et 3
PhCHO
Et 2
BOMe
NaBH 4
e.
(or enantiomer)
COOMe
OMe
Ph
f. then aq.
wrkp. H
cat.EtOH, 1 eq.
(EtO) 2
Br
g. OEt
Ph
OMe
Bn
BOTf,
Et 3
h.
then aq.
wrkp.
OMe
Bn
OMe
OMe
c. O
tBuOK
3
OMe
OMe
3
, then
2
2
+
OMe
OMe
EtOH
2
4
OMe
OMe
COOEt
COOEt
NaH,
cat. EtOH
aq. NaCl
heat
OMe
OMe
COOEt
1. TBSCl
imid.
(COCl) 2
then Et 3
d.
Me
Me
(this enantiomer)
Me
Bu Bu
Bn
c
Cy Cy
Me
Me
Me
NaBH(OAc) 3
Me
Me
Me
a. starting with
Cl
Me
Me
Cl
Ph
Et 3
Ph
LDA, then
PhCH 2
Br
c
(COCl) 2
then
Et 3
Bn
Bu Bu
Me
c
1. TBSCl, imid.
3. DMSO, (COCl) 2
then Et 3
Me
Me
Bu Bu
Ph
Me
c
Me
Me
c
Me
Me