













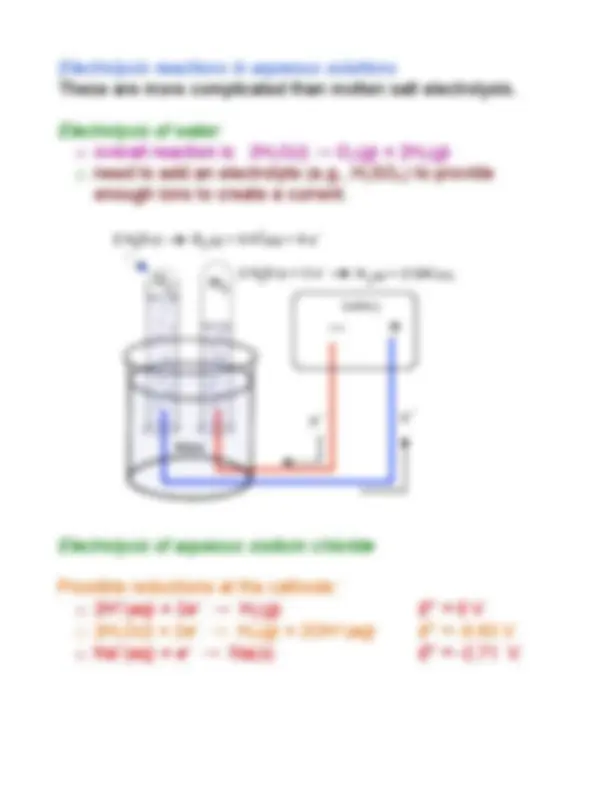





Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Measured current o called the overall cell potential (Ecell) o is the difference between the electrical potentials at the two electrodes (the two half-cell ...
Typology: Study notes
1 / 25

This page cannot be seen from the preview
Don't miss anything!


















Key topics:
Galvanic cells
Nernst equation
Batteries; electrolysis
Balancing Redox Reactions
A redox reaction involves a transfer of electrons from one species
to another. This results in a change in oxidation number.
Oxidation (reducing agent): loss of electrons
Reduction (oxidizing agent): gain of electrons
these reactions must occur together
example: thermal decomposition of potassium chlorate
� 2 ⇥ 3
� 1
0 ⇥ 2
example: combustion of methane
� 4
+1⇥ 4
0 ⇥ 2
� 2 ⇥ 2
+1⇥ 2
� 2
Balancing Simple Redox Reactions
In a redox reaction both the mass and the charge must be balanced
example: (net ionic equation)
Al( s ) + Ni
2+ ( aq ) → Al
3+ ( aq ) + Ni( s )
balanced by mass, but not by charge
What do we do?
half-reaction to balance the charge.
The two half-reactions correspond to reduction and oxidation
Al(s) → Al
3+ ( aq ) + 3 e
-
Ni
2+ ( aq ) + 2 e
- → Ni( s )
gained)
2 [Al( s ) → Al
3+ ( aq ) + 3 e
- ]
3 [Ni
2+ ( aq ) + 2 e
- → Ni( s ) ]
2 Al( s ) → 2 Al
3+ ( aq ) + 6 e −
3 Ni
2+ ( aq ) + 6 e − → 3 Ni( s )
2 Al( s ) + 3 Ni
2+ ( aq ) → 2 Al
3+ ( aq ) + 3 Ni( s )
Balancing Redox Reactions involving H and O
o you will be told if the reaction occurs under acidic or basic conditions o use H 2 O to balance oxygen o use H
to balance hydrogen o use OH
- to make basic (under basic conditions)
example: balance the following reaction (acidic conditions)
Cr 2 O 7
2 –
2+ → Cr
3+
3+
14H
2+ ! 2Cr 3+
Under basic conditions:
o balance as if under acidic conditions o for each H
in the final equation, add OH
and OH
example: balance the following reaction (basic conditions)
2 �
�
2 �
Balancing under acidic conditions gives
2 �
�
2 �
Then we add 2OH
2 �
�
2 �
�
Galvanic Cells
If we put a piece of copper metal into a AgNO 3 solution, a
redox reaction occurs: 2Ag
( aq ) + Cu( s ) → 2Ag( s ) + Cu
2+ ( aq )
The reaction will stop when the copper metal gets covered
with silver.
If we want to use this spontaneous reaction to extract energy
and do work we need to separate the two half reactions by
building a galvanic cell (also called a voltaic cell):
Cell Notation (shorthand for describing a galvanic cell):
Standard Reduction Potentials
Electrical current flows from the anode to the cathode due to a
difference in electrical potential energy between the electrodes
This current is measured, in volts (V), by a voltmeter.
Measured current
o called the overall cell potential ( E cell) o is the difference between the electrical potentials at the two electrodes (the two half-cell potentials) o varies with concentration, temperature, metals/ions used.
Standard cell potential : The cell potential under standard state
conditions, [ions] = 1 M , T = 25°C, 1 atm gas pressure.
Zn( s ) | Zn
2+ (1 M ) || Cu
2+ (1 M ) | Cu( s )
Each half-reaction can be considered to have a reduction
potential associated with it. This measures the natural
tendency of the half-reaction to proceed as a reduction.
Standard reduction potential ( E ° or E °red): Reduction potential
for a half-reaction under standard state conditions.
When two half-reactions are connected
o the one with the larger (more positive) E °red goes as a reduction
o the other one (less positive E °red) goes as an oxidation
with E °ox = - ( E °red)
�
� red
cathode
� ox
anode
We can only measure E cell so how do we get E °red and E °ox?
We select a specific half-cell as our reference (defined as
zero) and then use this one to get E°red for all other half-cells.
Cu 2+
� red, Cu2+/Cu +^ E
� ox, H+/H 2 H 2 (g)! 2H
Cu 2+
Pt( s ) | H 2 (1 atm) | H
(1 M ) || Cu
2+ (1 M ) | Cu( s )
How to measure the Zn
2+ standard reduction potential:
Measure the charge on the electrodes: H 2 (+) ⇒ cathode
Zn (–) ⇒ anode
Measure the current: 0.76 V
2H
� red, H+/H 2 +^ E
� ox, Zn2+/Zn Zn(s)! Zn 2+
2H+^ + Zn(s)! Zn2+^ + H 2 (g) E ox� , Zn2+/Zn = +0.76 V
E � red, Zn2+/Zn =^ �^0 .76 V Zn( s ) | Zn
2+ (1 M ) || H
(1 M ) | H 2 (1 atm) | Pt( s )
Once the half-cell potentials are determined, we can calculate E °cell E � cell =^ E
� red +^ E
� ox
E � cell =^ E
� red, Cu2+/Cu +^ E
� ox, Zn2+/Zn
Table 18.1: E ° values for several half reactions
more (+) : more likely to be reduced, better oxidizing agent
more (–) : more likely to be oxidized, better reducing agent
The Cl half-reaction will not occur because it would have to run as a reduction, but there is no Cl 2 ( g ) present. (a) The Pb half-reaction will not occur because it would have to run as a reduction, but there are no Pb
2+ ( aq ) ions present. (b) The Pb half-reaction would have to run as an oxidation and the hydrogen reaction would have to run as a reduction. This works because Pb( s ) and H
( aq ) are present. The reaction is
2H
( aq ) + Pb( s ) → H 2 ( g ) + Pb
2+ ( aq )
Spontaneity of Redox Reactions Under Standard State Conditions
ΔG is the maximum useful work that can be obtained.
In a galvanic cell, the work is supplied by the electric current (moving electrons through a wire)
�
� cell n = number of moles of electrons
F = Faraday constant = electric charge contained in one mole of electrons
E cell� measured in V
We can introduce the equilibrium constant K through
�
�
�
�
�
e.g. , Calculate Δ G ° for the following reaction at 25°C Pb( s ) + Ni
2+ ( aq ) Pb
2+ ( aq ) + Ni( s )
Solution: The relevant entries in Table 18.1 are:
Pb
2+ ( aq ) + 2e
Ni
2+ ( aq ) + 2e
The given reaction runs nickel as a reduction and lead as an oxidation reaction, thus E °cell = - 0.25 V + 0.13 V = - 0.12 V.
Since E °cell < 0 this reaction is not spontaneous and Δ G ° > 0.
�G
� = �nFE
� cell =^ �(2)(96500)(�^0 .12) = 23.16 kJ/mol
e.g. , Calculate the equilibrium constant for the following
reaction at 25°C 2Ag( s ) + Fe
2+ ( aq ) 2Ag
( aq ) + Fe( s )
Solution: The relevant entries in Table 18.1 are:
Ag
( aq ) + e
Fe
2+ ( aq ) + 2e
The given reaction runs silver as an oxidation and iron as a reduction, thus E °cell = - 0.44 – 0.80 = - 1.24 V.
� cell =^
n
log K ) log K =
nE cell�
0 .0592 V
� 42
e.g. , What is [Cu
2+ ] when a standard Ag/Ag
half-cell connected to a Cu/Cu
2+ half-cell (Cu electrode is negative) has E cell = 0.62 V?
Solution:
The reaction is 2Ag
( aq ) + Cu( s ) Cu
2+ ( aq ) + 2Ag( s ) and
Ag
( aq ) + e
Cu
2+ ( aq ) + 2e
Thus n = 2 and E °cell = 0.80 V – 0.34 V = 0.46 V, and
0 .62 V = 0.46 V � 0 .01285 ln Q ) Q = 3. 91 ⇥ 10 � 6 = [Cu 2+ ]/[Ag
] 2
Thus [Cu
2+ ] = 3.9 x 10
Concentration Cell : is a galvanic cell composed of the same material but differing in ion concentrations.
For a zinc concentration cell, the reduction half-reaction
Zn
2+ ( aq ) + 2 e
will be favored for high [Zn
2+ ( aq )]
and the oxidation half-reaction Zn( s ) → Zn
2+ ( aq ) + 2 e
will be favored at low [Zn
2+ ( aq )] from Le Chatelier’s principle.
Therefore the reduction takes place in the more concentrated
half-cell and the oxidation occurs in the more dilute half-cell.
For a zinc concentration cell of
Zn( s ) | Zn
2+ (0.10 M ) || Zn
2+ (1.0 M ) | Zn( s )
the cell potential is
The cell potential decreases during the operation of the cell
until the concentration of ions in the two compartments are
equal, at which point E = 0.
e.g. , Consider a copper concentration cell. One half-cell has
1.00 M CuNO 3 and the other contains a saturated solution of
CuCl. The cell potential is 0.175 V. Find Ksp for CuCl.
Solution:
We have 0.175 V = 0 – (0.0592 V) (log [Cu
]), Cu+^ of the CuCl
so [Cu
] = 1.11 x 10
2 = 1.22 x 10
Batteries
A battery is a galvanic cell, or a series of connected galvanic
cells, that can be used as a self-contained source of direct
electric current.
Lead acid battery
o made of six identical cells in series (each delivers 2 V) o lead anode and PbO 2 cathode immersed in H 2 SO 4. o rechargeable (use electrolysis) o discharge uses H 2 SO 4 and the solution density decreases (can measure density with a hydrometer)
Lithium-ion battery
o Ecell = 3.4 V, a relatively large potential o can be recharged hundreds of times
Fuel cells
o direct production of electricity (electrochemical process) o requires a continuous supply of reactants o very efficient o the only waste product is water!