


Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
The concept of atomic mass and how it is calculated using the masses and fractional abundances of naturally occurring isotopes. It provides examples and practice problems for calculating the atomic masses of various elements, including silicon, gallium, bromine, and titanium.
Typology: Lecture notes
1 / 2

This page cannot be seen from the preview
Don't miss anything!


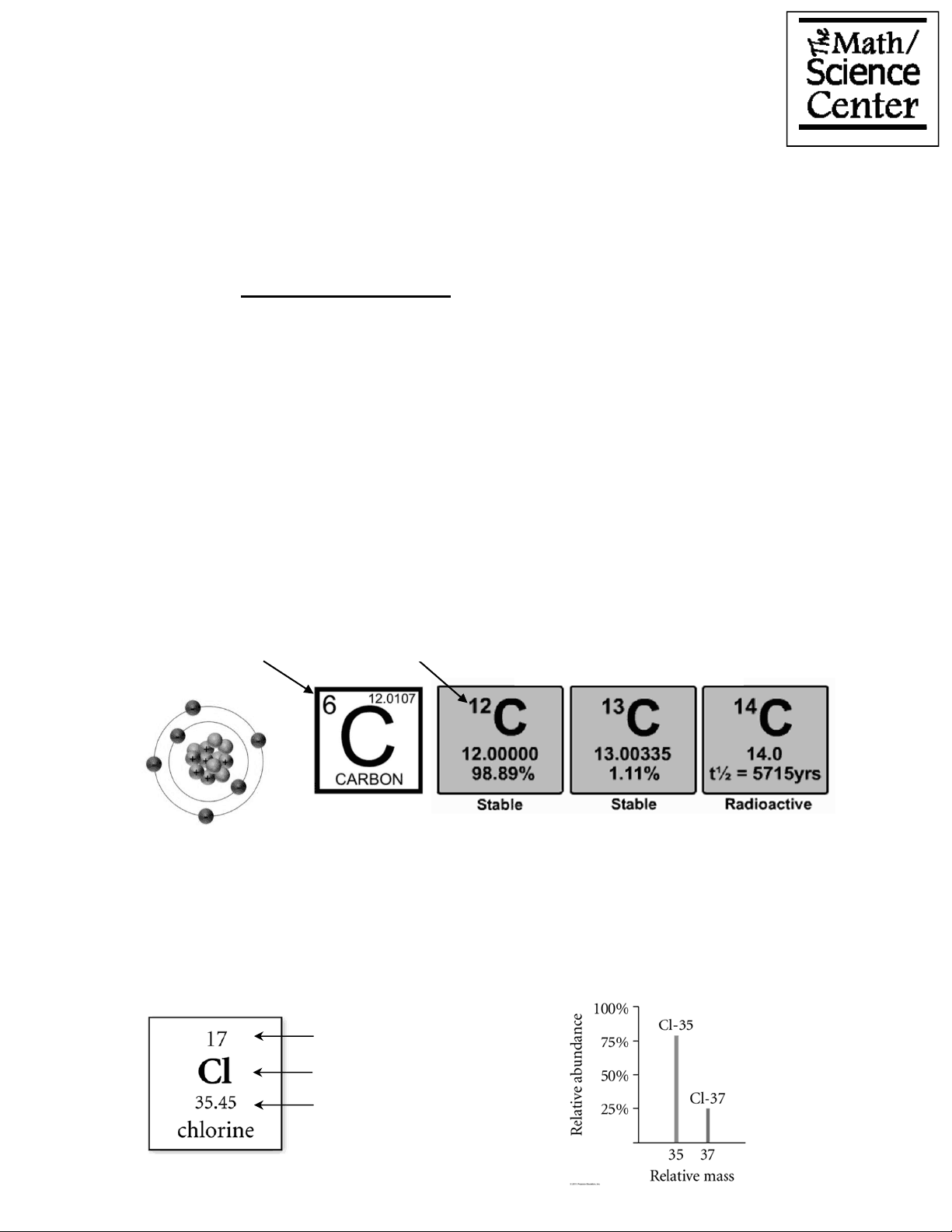
One atomic mass unit (amu) : the mass exactly equal to one-twelfth the mass of one carbon-12 atom that has six protons and six neutrons. 1 atom of carbon-12 = 12 amu
1 amu =
1 amu = 1.66054 x 10 -24^ g and 1 g = 6.02214 x 10^23 amu
Average Atomic Mass : the weighted average of the masses of the naturally occurring isotopes of the element; the mass of the atom in atomic mass units
Average Atomic Mass = ∑ (fractional abundance of isotope n) × (mass of isotope n)
Isotopes : atoms with identical atomic numbers but different mass numbers (that is, same number of protons but different numbers of neutrons)
Average atomic mass of carbon = (0.09890)(12.00000 amu) + (0.0110)(13.00335 amu) = 12.0107 amu
Example
If chlorine is 75.78 % Cl-35 with a mass of 34.9689 amu and the rest Cl-37 with a mass of 36.9659 amu , find chlorine’s atomic mass.
Cl atomic mass = 0.7578 x 34.9689 + (1 - 0.7578) × 36.9659 = 35.45 amu
Atomic Number
Atomic Symbol Atomic Weight
Carbon Isotopes
Atomic Number (number of protons)
Mass number (number of protons plus neutrons)
Atomic mass = (Fraction of isotope 1 x Mass of isotope 1) +
(Fraction of isotope 2 x Mass of isotope 2) +
(Fraction of isotope 3 x Mass of isotope 3) + ……
Practice Problems
Isotope Aundance (fraction) Atomic mass (amu) (^46) Ti 0.0825 45. (^47) Ti 0.0744 46. (^48) Ti 0.7372 47. (^49) Ti 0.0541 48. (^50) Ti 0.0518 49.
References: Tro, Chemistry: A Molecular Approach 2 nd^ ed., Pearson Brown/LeMay/Bursten, Chemistry: The Central Science, 12th^ ed., Pearson
Answers
47.7 amu 4. (a) 49.31% (b) 78.91 amu 3. 69.72 amu 2. 28.09 amu 1.