






Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Amino Acid Degradation Material Type: Notes; Professor: Joseph; Class: BIOCHEMISTRY; Subject: BIOLOGY; University: St. John's University-New York; Term: Fall 2011;
Typology: Study notes
1 / 17

This page cannot be seen from the preview
Don't miss anything!










Tymoczko • Berg • Stryer
© 2010 W. H. Freeman and Company
The First Step in Amino Acid Degradation Is the Removal of
The First Step in Amino Acid Degradation Is the Removal of
Nitrogen
Nitrogen
The major site of amino acid degradation in mammals is the
The major site of amino acid degradation in mammals is the liver.
liver.
The
The amino group must be removed
amino group must be removed
there are no nitrogenous compounds in energy-transduction
there are no nitrogenous compounds in energy-transduction
pathways.
pathways.
The α-ketoacids that result from the deamination of amino acids
The α-ketoacids that result from the deamination of amino acids
are metabolized so that the
are metabolized so that the carbon skeletons can enter the
carbon skeletons can enter the
metabolic mainstream
metabolic mainstream as precursors to glucose or citric acid cycle
as precursors to glucose or citric acid cycle
intermediates.
intermediates.
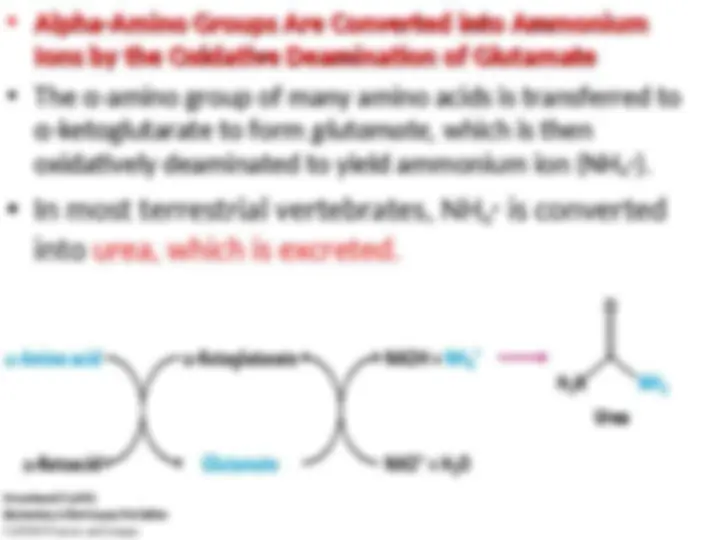
Aminotransferases
Aminotransferases catalyze the transfer of an
catalyze the transfer of an α-
α- amino group from an
amino group from an
α-
α- amino acid to an
amino acid to an α-
α- ketoacid. These enzymes, also called
ketoacid. These enzymes, also called
transaminases,
transaminases, generally funnel
generally funnel α-
α- amino groups from a variety of
amino groups from a variety of
amino acids to
amino acids to α-
α- keto-glutarate for conversion into NH
keto-glutarate for conversion into NH
44
++
Peripheral Tissues Transport Nitrogen to the
Peripheral Tissues Transport Nitrogen to the
Liver
Liver
tissues other than the liver.
tissues other than the liver.
muscle uses amino acids as a source of fuel
during prolonged exercise and fasting.during prolonged exercise and fasting.
the nitrogen must be released in a form that
the nitrogen must be released in a form that
can be absorbed by the liver and converted into
can be absorbed by the liver and converted into
urea.urea.
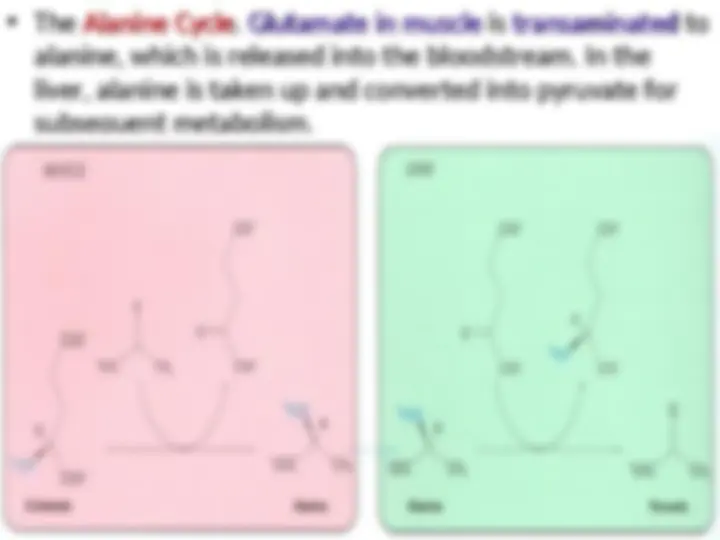
Nitrogen is transported from muscle to the liver
in two principal transport forms. Glutamate is
in two principal transport forms. Glutamate is
formed by transamination reactions, but the
formed by transamination reactions, but the
nitrogen is then transferred to pyruvate to formnitrogen is then transferred to pyruvate to form
alanine, which is released into the blood. Thealanine, which is released into the blood. The
liver takes up the alanine and converts it back
liver takes up the alanine and converts it back
into pyruvate by transamination.
into pyruvate by transamination.
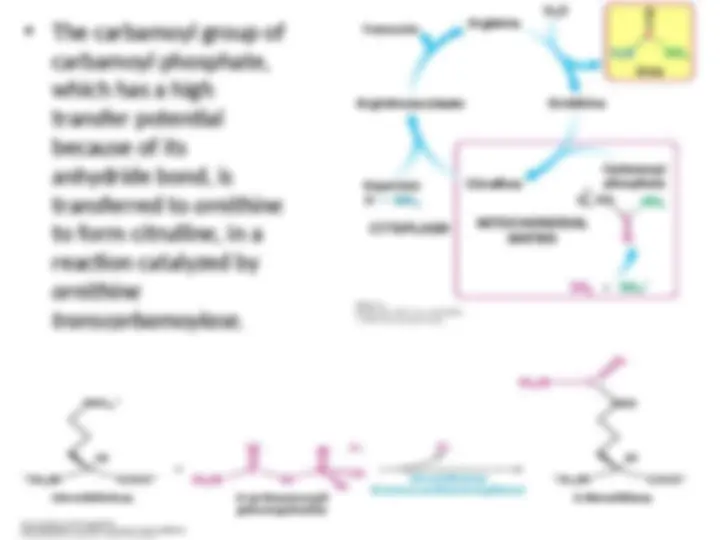
The carbamoyl group of
The carbamoyl group of
carbamoyl phosphate,
carbamoyl phosphate,
which has a high
which has a high
transfer potential
transfer potential
because of its
because of its
anhydride bond, is
anhydride bond, is
transferred to
transferred to ornithine
ornithine
to form
to form citrulline,
citrulline, in a
in a
reaction catalyzed by
reaction catalyzed by
ornithine
ornithine
transcarbamoylase.
transcarbamoylase.
Ornithine and citrulline are
Ornithine and citrulline are
amino acids, but they are not
amino acids, but they are not
used as building blocks of
used as building blocks of
proteins.
proteins.
Citrulline is transported to the
Citrulline is transported to the
cytoplasm where it condenses
cytoplasm where it condenses
with aspartate, the donor of
with aspartate, the donor of
the second amino group of
the second amino group of
urea. This synthesis of
urea. This synthesis of
argininosuccinate,
argininosuccinate, catalyzed by
catalyzed by
argininosuccinate synthetase
argininosuccinate synthetase
Finally, arginine is
Finally, arginine is
hydrolyzed to generate
hydrolyzed to generate
urea and ornithine in a
urea and ornithine in a
reaction catalyzed by
reaction catalyzed by
arginase.
arginase. Ornithine is then
Ornithine is then
transported back into the
transported back into the
mitochondrion to begin
mitochondrion to begin
another cycle. The urea is
another cycle. The urea is
excreted. Indeed, human
excreted. Indeed, human
beings excrete about 10 kg
beings excrete about 10 kg
(22 pounds) of urea per
(22 pounds) of urea per
year.
year.
Ammonium Ion
Ammonium Ion Is Converted
Is Converted
Into
Into Urea
Urea in Most Terrestrial
in Most Terrestrial
Vertebrates
Vertebrates
Some of the NH
Some of the NH
44
++
formed in the
formed in the
breakdown of amino acids is
breakdown of amino acids is
consumed in the biosynthesis
consumed in the biosynthesis
of nitrogen compounds. In
of nitrogen compounds. In
most terrestrial vertebrates,
most terrestrial vertebrates,
the excess NH
the excess NH
44
is converted
is converted
into
into urea
urea and then excreted.
and then excreted.
Such organisms are referred to
Such organisms are referred to
as
as ureotelic.
ureotelic.
One of the nitrogen atoms of
One of the nitrogen atoms of
the urea is transferred from an
the urea is transferred from an
amino acid, aspartate. The
amino acid, aspartate. The
other nitrogen atom is derived
other nitrogen atom is derived
directly from free NH
directly from free NH
44
, and the
, and the
carbon atom comes from HCO
carbon atom comes from HCO
33
(derived by hydration of CO
(derived by hydration of CO
2
2
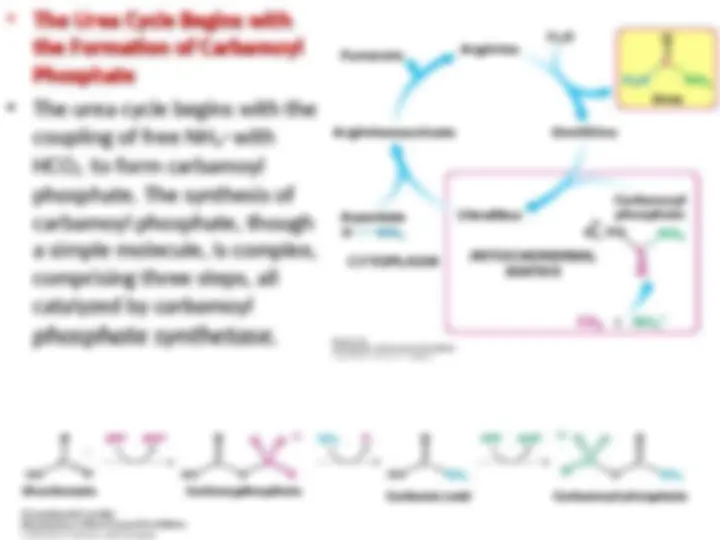
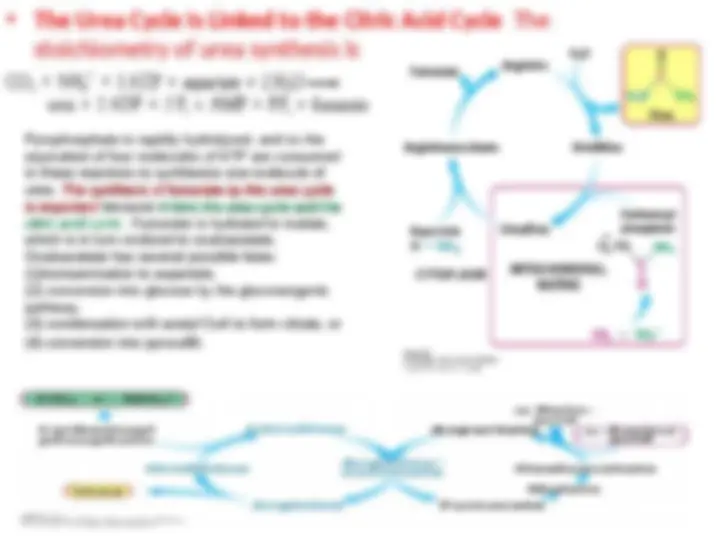
Metabolic Integration of Nitrogen Metabolism. The urea cycle, the citric acid
Metabolic Integration of Nitrogen Metabolism. The urea cycle, the citric acid
cycle, and the transamination of
cycle, and the transamination of oxaloacetate
oxaloacetate are linked by fumarate and
are linked by fumarate and
aspartate
aspartate.
Inherited Defects of the Urea Cycle Cause Hyperammonemia and Can Lead toInherited Defects of the Urea Cycle Cause Hyperammonemia and Can Lead to
Brain Damage
Brain Damage
The synthesis of urea in the liver is the major route of removal of NH
44
. A blockage . A blockage
of
of carbamoyl phosphate synthesis
carbamoyl phosphate synthesis or of any of the four steps of the urea cycle has
or of any of the four steps of the urea cycle has
devastating consequences devastating consequences because there is no alternative pathway for thebecause there is no alternative pathway for the
synthesis of urea.
synthesis of urea. All defects in the urea cycle lead to an
All defects in the urea cycle lead to an elevated level of NH
elevated level of NH
44
++
in
in
the blood (hyperammonemia
the blood (hyperammonemia ).
). Some of these genetic defects become evident a
Some of these genetic defects become evident a
day or two after birth, when the afflicted infant becomes lethargic and vomits
day or two after birth, when the afflicted infant becomes lethargic and vomits
periodically. Coma and irreversible brain damage may soon follow. Why are high
periodically. Coma and irreversible brain damage may soon follow. Why are high
levels of NH levels of NH
4
4
++
toxic? The answer to this question is not yet known. One possibilitytoxic? The answer to this question is not yet known. One possibility
is that elevated levels of glutamine, formed from NH
is that elevated levels of glutamine, formed from NH
44
and glutamate (,produce
and glutamate (,produce
osmotic effects that lead directly to brain swelling.
osmotic effects that lead directly to brain swelling.