


Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Material Type: Assignment; Class: General Chemistry II; Subject: Chemistry; University: South Carolina State University; Term: Unknown 1989;
Typology: Assignments
1 / 3

This page cannot be seen from the preview
Don't miss anything!


___ 1. Which of the statements concerning equilibrium is false? a. A system that is disturbed from an equilibrium condition responds in a manner to restore equilibrium. b. Equilibrium in molecular systems is dynamic, with two opposing processes balancing one another. c. The value of the equilibrium constant for a given reaction mixture is the same regardless of the direction from which equilibrium was attained. d. A system moves spontaneously toward a state of equilibrium. e. The equilibrium constant usually is independent of temperature.
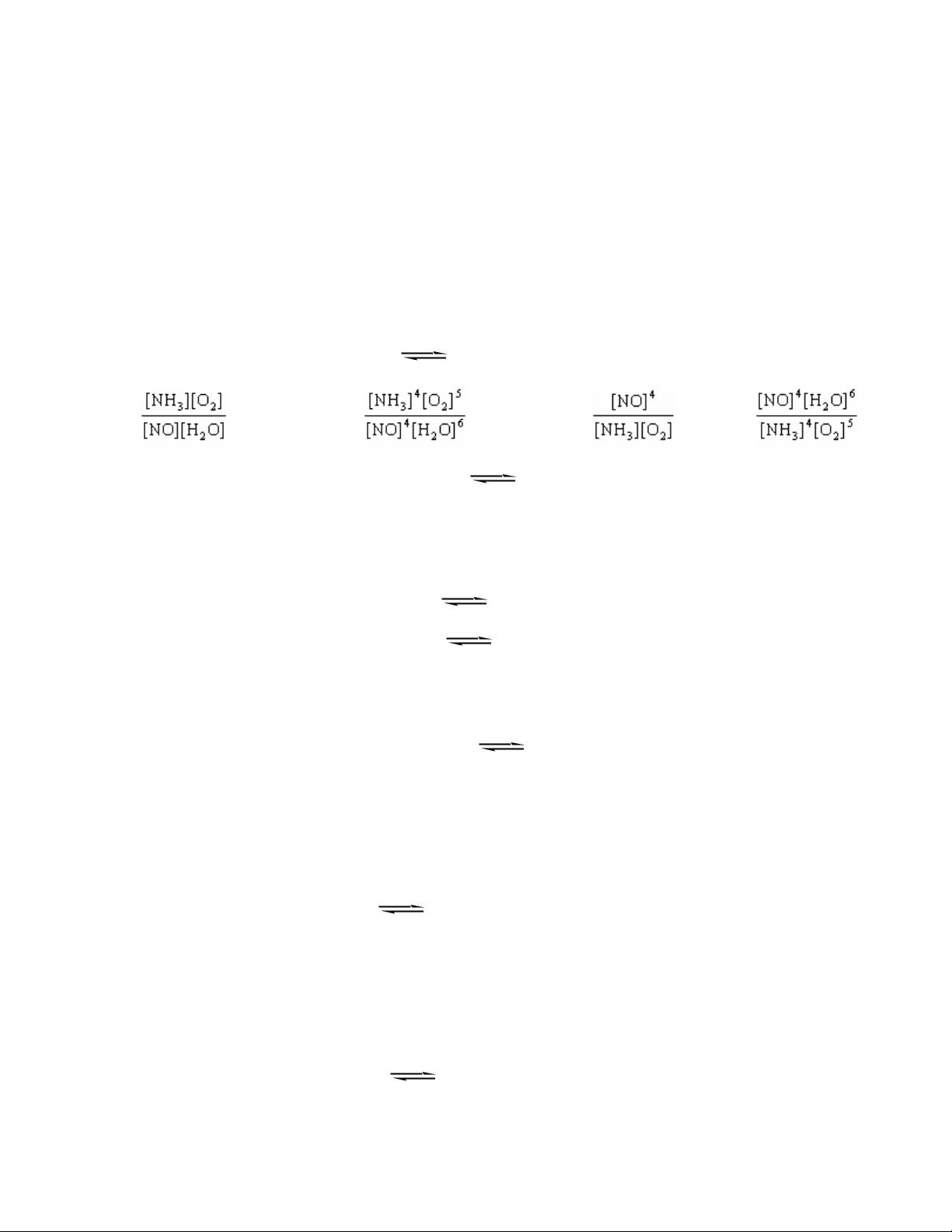
___ 2. Consider the gas phase system below at a high temperature. The form of the expression for the equilibrium constant, K c, __________. 4NH 3 + 5O 2 4NO + 6H 2 O a. cannot be determined without rate data
b. K c = c. K c = d. K c = e. K c =
___ 3. Nitrogen oxide and bromine combine to form nitrosyl bromide, NOBr. 2NO(g) + Br 2 (g) 2NOBr(g) In a 5.0 liter reactor at equilibrium at a constant temperature, it was determined there was 0.45 mol NO, 0.17 mol Br and 1.32 mol NOBr. What is the value of Kc at this temperature? a. 0.0116 b. 86.2 c. 253 d. 0.00395 e.22.
___ 4. If the equilibrium constant at a certain temperature is 2.1 × 1013 for the following reaction, 4HCl(g) + O 2 (g) 2Cl 2 (g) + 2H 2 O(g) calculate the value of the equilibrium constant at the same temperature for Cl 2 (g) + H 2 O(g) HCl(g) + O 2 (g). a. 2.2 × 10 -7^ b. 3.8 × 103 c. 5.3 × 1012 d. 4.7 × 10 -4^ e. 1.2 × 10 -
___ 5. The equilibrium constant, K c, for the following gas phase reaction is 0.50 at 600°C. A mixture of HCHO, H 2 , and
CO is introduced into a flask at 600°C. After a short time, analysis of a small amount of the reaction mixture shows the concentrations to be [HCHO] = 1.5 M , [H 2 ] = 1.2 M , and [CO] = 1.0 M. Which of the following statements about this reaction mixture is true? HCHO H 2 + CO
___ 6. Nitrosyl chloride, NOCl, dissociates on heating as shown below. When a 1.50-gram sample of pure NOCl is
heated at 350°C in a volume of 1.00 liter, the percent dissociation is found to be 57.2%. Calculate the equilibrium concentration of NOCl. NOCl(g) NO(g) + Cl 2 (g)
___ 7. The equilibrium constant for the following gas phase reaction is 4.0 at a certain temperature. A reaction is carried out at this temperature starting with 2.0 mol/L of CO and 2.0 mol/L of H 2 O. What will be the equilibrium concentration of H 2? CO + H 2 O CO 2 + H 2
Name: ……………… ___ 8. For the system H 2 (g) + CO 2 (g) H 2 O(g) + CO(g) at equilibrium, the addition of H 2 (g) would cause (according to LeChatelier's principle) a. only more H 2 O(g) to form. b. only more CO(g) to form. c. more H 2 O(g) and CO(g) to form. d. only more CO 2 (g) to form. e. no change in amounts of products or reactants.
___ 9. Which of the numbered responses lists all of the following stresses that would shift the equilibrium to the left (favor the reverse reaction), and no other stresses? 2NOCl(g) + 75 kJ 2NO(g) + Cl 2 (g) I. Add a catalyst. II. Heat the mixture. III. Decrease the volume at constant temperature. IV. Increase the partial pressure of NOCl by adding NOCl.
a. I, II, and IV b. II, III, and IV c. II and III d. III e. a different one or a different combination
___ 10. A system at equilibrium in a 1.0-liter container was found to contain 0.20 mol of A, 0.20 mol of B, 0.40 mol of C, and 0.40 mol of D. If 0.15 mol of A and 0.15 mol of B are added to this system, what will be the new equilibrium concentration of A? A(g) + B(g) C(g) + D(g)
___ 11. A mixture of 0.40 mol of N 2 , 0.60 mol of Ar, and 0.30 mol of O 2 is confined in a 300-liter vessel at 50.0°C. What is the total pressure of the system?
___ 12. The equilibrium constant expression for the reaction, 2NO 2 (g) O 2 (g) + 2NO(g) is given by K p =
___ 13. K c = 4.6 × 10 -3^ for the reaction below at 25°C. Evaluate K p at 25°C. N 2 O 4 (g) 2NO 2 (g)
___ 14. The equilibrium constant, K p, for the following reaction is 280. at 150.°C. Suppose that a quantity of IBr is placed
in a closed reaction vessel and the system is allowed to come to equilibrium at 150.°C. When equilibrium is established, the pressure of IBr is 0.200 atm. What is the pressure of I 2 at equilibrium? I 2 (g) + Br 2 (g) 2IBr(g) + 11.7 kJ