


















































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Crystal Field Theory Material Type: Notes; Professor: Megehee; Class: ADVANCED INORGANIC CHEMISTRY; Subject: CHEMISTRY; University: St. John's University-New York; Term: Fall 2011;
Typology: Study notes
1 / 65

This page cannot be seen from the preview
Don't miss anything!


























































(^) Justified using MO theory
(^) To obtain Noble gas e^ configuration need 18 e 's (^) Hence 18 e^ Rule!!
How Many Electrons Does Each Ligand Donate?
, :Cl , :Br , :I , :H , :CH 3 , :CR 3 , :SH , :SR (^) :CN: , :CO:, :OH , :OR , :OH 2
(^) :NH 3
3
3 , :AsR 3
H 2 N NH 2 R 2 P^ PR (^2) AsR R 2 As (^2) C C O O O O N N N N N NH NH H 2 N 2
(^) Add or subtract charge from metal to get M oxidation state.
(^) Break homolytically—give 1 e^ to each M (^) Count M—M as 1 e^ donor (^) as 2 e^ donor (^) as 3 e^ donor (^) as 4 e^ donor M M M M M M
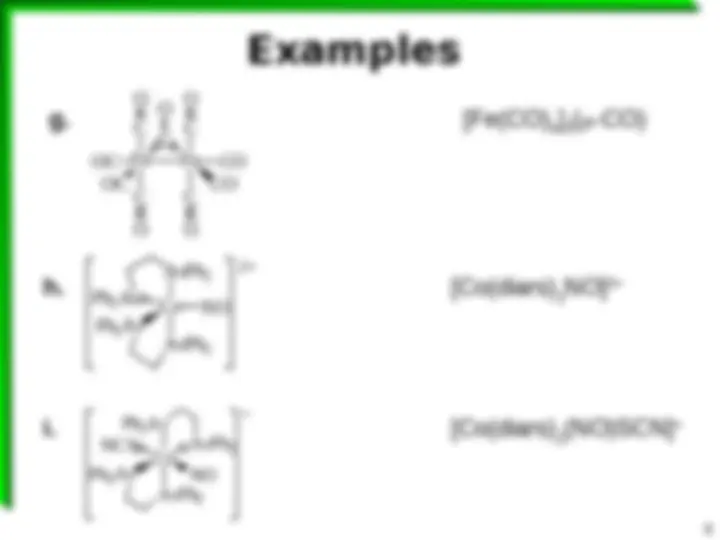
Cr OC OC CO CO CO CO a. Cr d 6 6 CO 6 e 12 e 18 e Cr(CO) 6 C Re PMe 3 CO CO CO O H 3 C b. (^) Re(PMe 3 ) (CO) 3 (COCH 3 ) Re O O N N N N
CN NC Co CN CN c. (^) [Re(py) 4 (O) 2 ]
d. (^) [Co(CN) 4 ]
Co NO Ph 2 As Ph 2 As AsPh 2 AsPh 2 2+ Co Ph 2 As NCS AsPh 2 NO Ph 2 As AsPh 2
h. [Co(diars) 2 NO] 2+ i. [Co(diars) 2 (NO)SCN]
Fe Fe C C C C OC CO OC CO C O O O O O g. [Fe(CO) 4 ] 2 (-CO)CO)
(^) H•, Cl•, Br•, I•, R•^ (R = alkyl or phenyl), or RO•
3
3
2
2
2 -alkene), Ralkene), R 2
(carbene, M=CR 2
3 -alkene), RC 3
5
, -alkene), R R 2
(^) ^4 -alkene), Rdiene; ^4 -alkene), RC 4
4 (cyclobutadienes) H 2 C H C CH 2
5 -alkene), RC 5
5
6 -alkene), RC 6
6 (benzene or other 6 -alkene), Rarenes e.g. 6 -alkene), R C 6
5 Me)
(^) 3 e– (^) 1 e–
o
(^) Rigorously conform to 18 e^ Rule (^) t 2g level is bonding now, so energetically favorable to have completely filled (^) TM-alkene), RCarbonyl and -alkene), Rbonding organometallic compounds
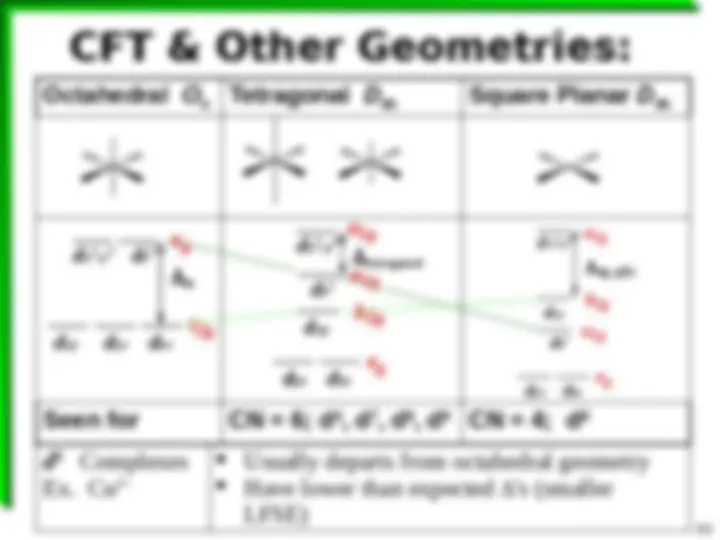
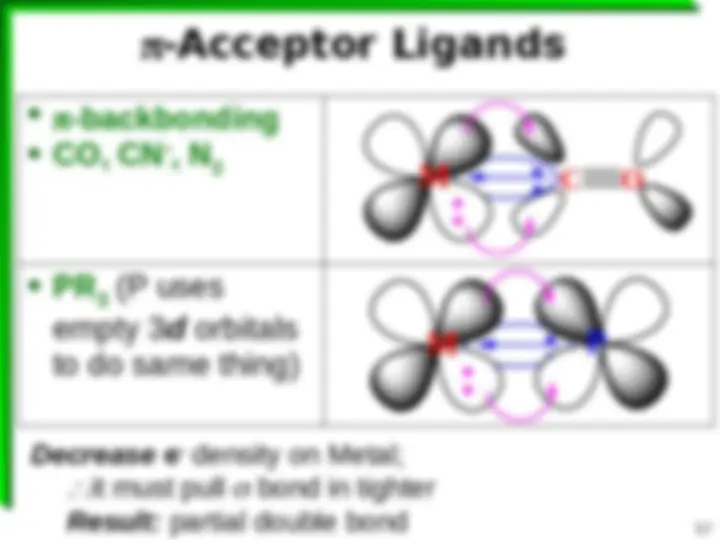
18 e Rule Not Rigorously Followed Complexes with d 8 e configurations (^) Sometimes 18 valence e's strongly -alkene), R backbonding ligands (^) Removes e^ density from M (^) Fe(CO) 5 , Fe(CNR) 5 , [Pt(SnCl 3 ) 5 ] 3 (^) Sometimes 16 valence e's NO -alkene), R backbonding ligands (^) Doesn't remove e^ density from M (^) [AuCl 4 ] , [PdCl 4 ] 2 , Ni(dmg) 2 Note: explains why some Metals favor CN = 4 Some CN = 5 Some CN = 6, etc.
Bonding & Electronic Structure in Transition Metal Complexes
(^) Simplest (^) Purely Electrostatic (ionic) model (^) Ignores covalent bonding interactions with TM (^) Ligand lone pair = point negative charge (^) Repels e 's—s in d orbital on TM (^) Allows us to understand & correlate all those properties that arise from presence of partly filled shells
Bonding & Electronic Structure in Transition Metal Complexes
(^) Extension of MO theory (^) More exact (^) Includes both covalent and electrostatic interaction between ligands and TM (^) Focus: role of d orbitals on TM overlap with & ligand orbitals (^) More cumbersome
1. Place TM ion in spherical ligand field of negative charge = 12 e 's—s Fe 3+ ( d 5 ) free atom/ion spherical negative charge
3 + 12 e
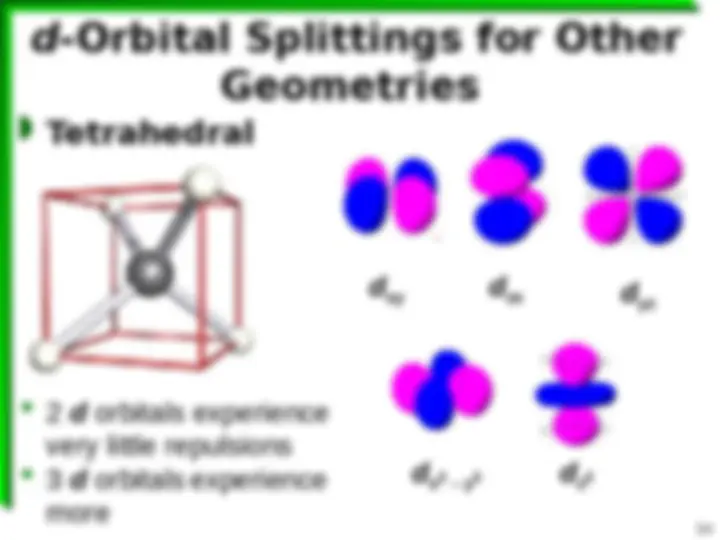
(^) Come in along x , y , & z axes
(^) Incoming lone pairs on ligands are pointing directly toward d orbitals containing e 's—s.
xy
yz
zx (^) Incoming lone pairs on ligands pointing in 2 2 x y d 2 z d